Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.20 no.1 Madrid ene./feb. 2005
Revisión
Diet and colorectal cancer: current evidence for etiology and prevention
F. G. Campos, A. G. Logullo Waitzberg, D. R. Kiss, D. L. Waitzberg, A. Habr-Gama and J. Gama-Rodrigues
Department of Gastroenterology. Colorectal Surgery Unit. Hospital das Clínicas. University of São Paulo Medical School.
| Abstract The etiology of colorectal cancer (CRC) involves the interaction of cell molecular changes and environmental factors, with a great emphasis on diet components. But the paths connecting lifestyle characteristicas and the colorectal carcinogenesis remain unclear. Several risk factors are commonly found in western diets, such as high concentrations of fat and animal protein, as well as low amounts of fiber, fruits and vegetables. A large number of experimental studies have found a counteractive effect of fiber on neoplasia induction, especially in relation to fermentable fiber (wheat bran and cellulose). Epidemiological correlation studies have also indicated that a greater ingestion of vegetables, fruit, cereal and seeds is associated to a lower risk for colorectal neoplasia. Moreover, beneficial properties of fiber (especially from vegetable sources) were documented in more than half of case-control studies. Nevertheless, recent epidemiological data from longitudinal and randomized trials tended not to support this influence. Future research should evaluate what sources of fiber provide effective anti-neoplasic protection, carrying out interventional studies with specific fibers for longer periods. Red meat, processed meats, and perhaps refines carbohydrates are also implicated in CRC risk. Recommendantions to decrease red meat intake are well accepted, although the total amount and composition of specific fatty acids may have distinct roles in this setting. Current evidence favors the substitution of long and medium-chain fatty acids and arachidonic acid for short-chain fatty acids and eicosapentaenoic acid. Excess boy weight and excess energy intake inducing hyperinsulinemia have been also associated to CRC, as well as personal habits such as physical inactivy, high alcohol consumption, smoking and low consumption of folate and methionine. Thus, current recommendations for decreasing the risk of CRC include dietary measures such as increased plant food intake; the consumption of whole grains, vegetables and fruits; and reduced red meat intake. (Nutr Hosp 2005, 20:18-25) Key words: Colorectal cancer, epidemilogy, risk factors, fiber.
| DIETA Y CÁNCER COLORRECTAL: EVIDENCIA ACTUAL SOBRE LA ETIOLOGÍA Y LA PREVENCIÓN Resumen La etiología del cáncer colorrectal (CCR) implica la interacción entre los cambios celulares moleculares y los factores ambientales, con un gran énfasis sobre los componentes de la dieta. Pero los caminos que conectan las características del estilo de vida con la carcinogénesis siguen siendo inciertos. En las dietas occidentales se encuentran, habitualmente, diversos factores de riesgo como las concentraciones elevadas de grasa y proteínas de origen animal, así como cantidades bajas de fibra, frutas y vegetales. Un gran número de estudios experimentales han encontrado que la fibra contrarresta la inducción de neoplasia, especialmente en relación con la fibra fermentable (salvado de trigo y celulosa). Los estudios de correlación epidemiológica también han indicado que una mayor ingestión de vegetales, frutas, cereales y semillas se asocia con un riego menor de neoplasia colorrectal. Además, en más de la mitad de los estudios de casos-control, se documentaron las propiedades beneficiosas de la fibra (especialmente de origen vegetal). Sin embargo, los datos epidemiológicos recientes de estudios longitudinales y de distribución aleatoria no tendían a apoyar esta influencia. La investigación futura debería evaluar qué fuentes de fibra proporcionan una protección antineoplásica realizando estudios de intervención con fibras concretas, durante periodos más prolongados. Las carnes rojas y las procesadas, y quizás los hidratos de carbono refinados, también están implicadas en el riesgo de CCR. Están bien aceptadas las recomendaciones para disminuir la ingestión de carne roja, aunque la cantidad total y la composición de ácidos grasos concretos pueden tener efectos distintos en este contexto. La evidencia actual se decanta por la sustitución de los ácidos grasos de cadena larga y media y del ácido araquidónico por los ácidos grasos de cadena corta y por el ácido eicosanopentanoico. El exceso de peso corporal y el exceso de aporte de energía que induce una hiperinsulinemia también se han relacionado con el CCR, así como los hábitos personales como la inactividad física, el consumo elevado de alcohol, el tabaquismo y el consumo bajo de folatos y metionina. Por lo tanto, las recomendaciones actualespara disminuir el riesgo de CCR incluyen medidas dietéticas como aumentar los alimentos de origen vegetal, el consumo de granos completos, vegetales y frutas y reducir el consumo de carnes rojas. (Nutr Hosp 2005, 20:18-25) Palabras clave: Cáncer colorrectal, epidemiología, factores de riesgo, fibra. |
Correspondencia:
F. G. Campos
Departmen of Gastroenterologoy, Colorectal Surgery Unit
Hospital das Clínicas, University of São Paulo Medical School
Rua Padre João Manuel, 222
São Paulo, Brazil
E-mail: fgcampos@osite.com.br
Recibido: 20-V-2004.
Aceptado: 20-VII-2004.
Introduction
Although colorectal cancer (CRC) exhibits universal distribution, there is a higher incidence if the disease in developed, industrialized countries, such as North America, Northern Europe and New Zealand. The disease is less common in South America, Southeast Asia, Equatorial Africa and India51.
In the United States, these tumor arte the second most prevalent neoplasia group among men (following lung cancer) and the third among women (following breast cancer and lung cancer). In 2003, around 147 thousand new cases of CRC were diagnosed and 57 thousand people died from the disease. According to estimates by the National Cancer Institute (INCA) for 2003 in Brazil, around 20 thousand new cases were diagnosed, leading to death in 8 thousand patients.
The epidemilogy of CRC has aroused interest in recent years as a result of developments in genetics and molecular biology. The genetic alterations that lead to CRC may either be acquired (generating the so-called sporadic cancer) or hereditary. During this process, the accumulation of genetic alterations is essential and mutations in at least 4 or 5 genes are necessary for the development of a malignant tumor. The vast majority (75 to 85%) of patients have sporadic CRC, exhibiting no evidence of a genetically inherited disease in which the risk of developing CRC is high.
Nowadays it is well recognized that a complex interaction between individual gentic features and environmental factors, especially diet, is involved in the etiology of CRC. Therefore, prevention depends on dietary education as well as on tracking high-risk groups and treatment of pre-malignant lesions.
That worldwide of incidence and mortality of the disease suggest a co-existence of environmental causes of great impact, and studies on migran populations suggest that the risk for CRC is influenced by environmental exposure50. Environmental mutagenic factors may determine which susceptible individuals will develop carcinoma. Thus, there is considerable interest in defining strategies and recommendations for preventing the development of CRC by modifications in eating habits and life style (smoking, sedentarism, etc.).
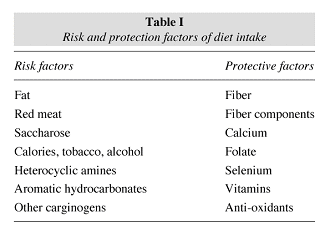
Among dietary properties, western diet stands out for being rich in fats, animal proteins and calories, as well as poor in fiber (fruits, vegetables and cereals). Red meat, processed meats and refined carbohydrates contribute toward a higher risk29. From an environmental and behavioral perspective, risk factors also include a sedentary lifestyle, smoking, obesity, alcohol consumption, and ingestion of heterocyclic amines and aromatic hydrocarbonates29. On the other hand, protective agents include calcium, vitamin D, folates, selenium, vitamins and anti-oxidants. In general, these are the main factors associated with CRC, though other elements may also influence its genesis (table I).
In summary, diet can influence the genesis of CRC though detrimental effects (fats), chemical preventive (fibers) and endocrines mechanisms (calories), among others. Thus, the present article aimed to review some mechanism of CRC carcinogenesis associated to specific diets, to describe the action of some nutrients over the colonic mucosa, to present evidence of chemical preventative effects associated to nutrition and to elaborate dietary recommendations for the prevenion of CRC.
The role of fiber
The high consumption of saturated fats and animal protein associated with low fiber intake has traditionally been the main dietary characteristics implicated in CRC genesis. In this context, the elucidation of fiber action on the colon epithelium is important since it can suggest means for preventative interventions in the future. These mechanisms involve physical events (alteration of intestinal transit time, dilution of fecal bolus, physical or chemical adherence to mutagenicagents) and secondary effects (generation of bacterial fermentation products -especially short-chain fatty acids- and alteration of the luminal pH)28,42.
Thus, fiber deficiency increases intestinal transit time and raises the concentration of luminal contents, allowing a longer contact of the colonic mucosa with harmful and carcinogenic agents. Among such agents, fatty acids metabolities (bile salts) are generated by the metabolism of animal fat and protein. These elements lead to important epithelial alterations that may develop neoplasic cells in the colon13.
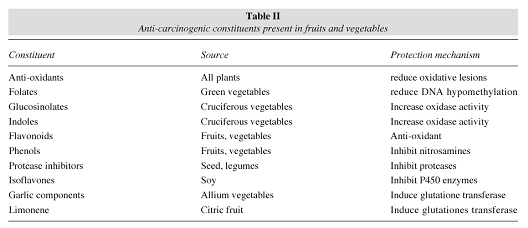
Some vegetables may provide greater benefits for the prevention of CRC, such as broccoli, cauliflower and brussel sprouts. These food sources contain high levels of sulphoraphan, elements that induce detoxification enzymes that increase the aqueous solubility of corporeal toxins and their subsequent elimination. More recently, it has been pointed out that various constituents found in fiber sources (especially fruits and vegetables) perform metabolic actions with anticarcinogenic effects (table II).
An increase in dietary fiber also raises the concentrations of short-chain fatty acids (SCFA) formed through bacterial fermentation. These products have an important role in colon metabolism especially butyrate. It has been demonstrated induction of cell lineages differentiation, trophic effects on the epithelium, intracellular anti-neoplasic action and inhibiton of hyper-proliferative epithelial growth11.
Furthermore, soluble fibers may delay starch absorption, reducing the glycemic load and consequent post-prandial hyperinsulinemia, which is linked to colon carcinogenesis22.
In 1969, Burkitt10 verified that the incidence of cancer among rural black Africans was lower than that amogn westerners who ingested a more processed diet. The author postulated that diet had an effect on the greater incidence of CRC among the white population, and that less processed foods and a higher amount of fiber had a protective effect on the black population. Since these original considerations, epidemiological, observational and interventional studies have collected diverse data regarding the relation between fiber ingestion and CRC.
Historically, a large number of epidemiological observations involved ethnic groups that had migrated countries where food consumption habits differed from those in their country51. The evolution of CRC incidence in African black that migrated to the USA has become a classic citation in the literature. In the USA, a diet rich in fat and poor in fiber increased significantly the CRC mortality, going from 2.5/100 thousand inhabitants, figures that are immensely superior to those found among Asian peoples. Other studies in Israel have also shown that the incidence of cancer among Jewish descendents of Americans and Europeans is higher than the average of the rest of the population who have distinct eating habits.
Literature data regarding fiber and CRC exhibits a huge body of evidence favoring its consumption. In an excellent review, Shankar and Lanza43 found 40 studies from 1980 to 1991 that evaluated this relation. Thirty-two of them demonstrated an inverse relation between a higher consumption of fiber and the incidence of tumor. According to an observational epidemiological study that grouped a sample of 519,978 adults in 22 urban centers of Europe showed that an increase in diet fiber content reduced the risk of CRC by 25%6. The supposed protective effects of fiber have also been recognized in meta-analyses26,48 and other review studies25,49.
Case-control and longitudinal epidemiological studies offer most of the evidence concerning fiber and CRC, because they are easier to carry out than interventional experiments. Two meta-analysis uniting 13 and 20 case-control studies demonstrated that diets rich in fiber reduce the risk of this cancer15,26. According to Howe and cols.26, the risk of colon cancer in the United States could be reduced by 31% if there were an ingestion of 25g of fiber per day. Other metaanalysis of 12 case-control studies selected by Trock and cols.48 pointed out the advantages of a greater ingestion of fiber in comparison to a lower consumption. A recent, large review confirmed that case-control studies lend support to the protective effects of high concentrations of fibers against colorectal neoplasias51. Peters and cols.37 also demonstrated that fiber (especially seeds, cereals and fruit) is associated to a lower risk of colon adenomas in more than 30 thousand patients.
In case-control studies, patients with CRC are identified in a given period of observation and are paired with demographic controls from the population. Previous exposure of the colon mucosa to dietary elements is quantified by means of inquiries into eating habits and food composition tables. Next, the odds ratio is calculated, measuring the possibility of developing the disease after exposure to a variable risk and comparing it to the base risk. However, the greatest problem with this type of study is the error that arises when patients with cancer modify their perception of their dietary habits. As a result, the study no longer reflects the real situations to which the individual were exposed50.
Nevertheless, the literature data are not unanimous in regards to the supposedly protective effects of fiber against CRC. While epidemiological and experimental studies unite favorable evidence, a number of longitudinal prospective studies and interventional series have not confirmed the idea. It should be pointed out, however, that the possible beneficial effects have not yet been attested due to the imprecision of the dietary ingestion measurements, the small number of patients and short periods of study20,42.
Longitudinal studies begin with the recruitment of a large number of volunteers, called cohort. The ingestion of fiber is quantified through inquires and foos composition tables. The cohort is accompanied in a systematic and prospective fashion, and the cases of adenomas, precursor lesions and colorectal cancer are identified. The critical point is that the information from the dietary inquiries becomes progressively less up-to-date over time and there is the further possibility of participants abandoning the study before its conclusion50.
The Nurses Health Study16 was developed over a period of 20 years. It assessed dietary exposure through the use of semi-quantitative questionnaires and standard food composition tables. The relative risk of CRC or adenomas demonstrated no differences among the five levels of ingestion of dietary fiber (9.8 to 24.9 g/day). Although there was a risk reduction with the ingestion fiber present in fruit (without any statistical significance), the consumption of vegetable fiber was surprisingly associated to a higher risk.
In USA, a prospective study with a cohort of nearly 16,500 men38 found no significant association between the ingestion of fiber (total, cereal or vegetable) and the appearence of adenomas, though there was a (nonsignificant) reduction with a greater consumption of gruit fiber. In another important research27 (Iowa Women's Health Study), the total ingestion of fiber was not associated with the risk of CRC. This was also the conclusion of a national tracking study carried out in Norway17.
Moreover, although the consumption of vegetables has been associated to a lower risk of adenomas and cancer in western patients5, results of the association of CRC with vegetables are as yet inconsistent in oriental countries33.
An explanation for the conflicting results between case-control and longitudinal studies may lie in the fact that the types of fibers differ in their effects on increasing fecal mass, altering the intestinal transit time and promoting fermentation. Furthermore, as few new cases of CRC were identified during longitudinal studies, it is possible that the lack of significant difference may be a consequence of statistical error.
A large number of interventional studies (both on animals and humans) have also addressed the issue. In an elegant experiment, Galloway and cols.19 demonstrated that the risk of developing CRC in mice depends on the type of diet employed, varying from 3.6% (low-fat, high fiber content diet) to 64% (employing a diet with a high fat content and low in fiber). Despite some limitations, animal models of colorectal neoplasia are very important to investigate the effects of dietary factors on carcinogenesis, but experimental results cannot be directly extrapolated to humans. Besides the diet and gastrointestinal tract in mice being distinct from humans, the carcinogens used to deflagrate the malignant neoplasia are not the same. Thus, many of the conflicting results found in the literature may be explained by the differences between natural history of tumors in humans and the development of experimental tumors.
In general, protective effects have been demonstrated, especially with fiber sources that are not particularly fermentable, such as a cellulose and wheat germ, whereas other soluble fibers generally increase CRC carcinogenesis28.
Considering the importance of secondary bile acids in the pathogenesis of colon cancer, the modulating effect of different types and quantities of fiber have been studied in relation to the type of dietary fat in healthy human volunteers. Reddy and cols.39 verified that a dietary supplementation of 10 grams of wheat and rye germ (27 grams of fiber per day) determined a significant reduction in fecal bile acids, especially secondary acids, during the period of fiber ingestion when compared to the control period without fiber.
In an interventional study carried out on patients that had undergone operations for colon cancer35, the offer of 20 grams of fiber from psyllium seed powder (plantago ovata) per day for three months promoted an increased production of butyrate and acetate. The concentration of fecal butyrate increased 42%, an effect that was dependant on the continuation of the treatment.
Controlled randomized studies are data sources of considerable interest. However, in dietary estudies, the different base diets make comparisons difficult between intervention and control groups. Most of the available studies were limited (from two to four years) and included small population samples taken from high-risk groups.
The so-called adenoma-carcinoma sequence is the conceptual basis of a number of interventional studies that have investigated the preventive potential of diet among individuals with a greater risk for cancer, such as a patients with polypops and adenomas. After polupectomy, patients present a 50% chance of developing additional polyps within a period of three years. In this setting, nutritional intervention studies have not demonstrated reduction in the development of further polyps (Phoenix Colon Cancer Prevention Trial2, Polyp Prevention Trial41).
In the Wheat Bran Fiber Study2, 1,429 individuals from the Phoenix area (USA) were randomized to receive cereal supplements (13,5 vs. 2 grams of fiber per day) for 3 years after having polyps removed in the previous 3 months. Colonoscopy performed after 1 and 4 years demonstrated no differences between the twho groups. The same occurred in an analysis of 2,079 patients with previously resected adenomas, in whom Schatzkin and cols.41 compared the use of a diet rich in fiber and poor in fat to the use of the habitual diet. Other series also obtained statistically non-significant reduction in the development of adenomas (Australian Polyp Prevention Project31, Toronto Polyp Prevention Trial32).
These studies demonstrated that an increased ingestion of fiber among high-risk populations for short periods of time did not reduce the recurrence of adenomaous polyps. Nevertheless, interpretation of these data requires many considerations. It is known that the natural history of CRC is a long duration process, where the evolution of one tiny polyp can take decades. Given enough time, the ingestion of fiber over a longer period and the evaluation during a greater follow-up could detect differences on the studied population. But the current interventional studies have not examined this aspect yet.
Individual susceptibility to colorectal neoplasias varies, as the presence of a personal and family history increases this risk. However, the stratification of this risk is yet imprecise. Thus, although controlled randomized studies with fiber and CRC involving high-risk populations have not demonstrated any significant effect, there must be subrgroups of individuals that are more susceptible to dietary manipulation.
Specific genetic mechanisms may be responsable for the malignant transformation of polyps, since just 5 to 10% of these adenomas may become cancer when not removed. Considering the possibility that different diets may have distinct effects on the molecular mechanisms within the polyps, future studies should evaluate the manipulation of diet on this particular group of polypoid lesions.
Finally, the effects of fiber on colorectal carcinogenesis cannot be examined separately. It is now recognized that vegetables and other agents such as vitamins and anti-oxidants have important benefits, and different types of fiber may have either synergic1 or antagonistic24 effects on colorectal neoplasias. Despite the controversy regarding protection from CRC, the current recommendations on increasing the average fiber ingestion of the population are sensible in that there is the recognition of the fiber effects on improvin overall health, reducing the risk of heart disease, hypertension, hypercholesterolemia, diabetes and other chronic illnesses34.
Thus, one must consider that the generic grouping of all types of fiber as "dietary fiber" may mask or confound its potential biological effects42.
Influence of fat
For millions of years, food from vegetal sources was the basis of human diet. With the advent of the industrial revolution 200 years ago, dietary habits in industrialized nations began to change through the refinement of food sources containing a large amount of fiber along with an increase in fat consumption. In this context, meat became a symbol of opulence among members of society. Subsequently, this greater amount of elements from animal origins increased the incidence of many illnesses such as a cancer, heart disease, diabetes and others.
Diverse evidence suggests that fat in the diet is associated to a greater risk of CRC. In countries with high incidence of CRC, the fat content in the diet represents about 40% of the total calories. This contrasts with low incidence regions where the fat content is just 15 to 20% of total calories33.
The quantity and composition of specific fatty acids influence this risk36. There is a strong association between CRC and the consumption of red meat (beef, lamb, pork and processed meats such as sausage, hamburger, ham and bacon). It has been suggested that this increased risk is due to the greater production of bile acids, formation of carcinogenic agents and toxic effects inducing the proliferation of colonocytes29.
There exists only scant associations between diet and the risk of a progressive accumulation of genetic damatge. Nitrous components (nitrosamins) are found in foods that contain nitrates or that have been exposed to nitrous oxide, such as processed meats8. Heterocyclic amines are formed on the surface of the meat when it is cooked over a direct flmae or at high temperatures and are activated metabolically. It is believed that local bioactivation of heterocyclic amines in the colon contributes to the development of cancer, inducing mutations in the APC and K-ras genes29,46.
In a systematic review about the risk of CRC and red meat, Sandhu and cols.40 included prospective cohort studies and excluded case-control and ecological studies, grouping a total of 601,000 participants and 3,617 cases. They observed that a daily increase of 100 grams of meat was associated to a 14% increase in CRC risk. Also, a daily increase of 25 grams of processed meat (cured or with nitrates) was associated to a 49% greater risk. The authors observed that the independent effect of meat consumption on CRC risk was evaluated in just a small number of the studies.
Thus, the limitation total fat consumption may be important in cancer prevention. There is a recommendation to limit the ingestion of red meat below 80 grams per day, especially for those individuals with a high CRC risk (genetic diseases and certain life style factors). Limiting the consumption of processed meat is especially advised, replacing it with fowl, fish, beans and legumes29.
In a recent review, Nkondjock and cols.36 demonstrated that high concentrations of short-chain fatty acids (SCFA) and eicosapentaenoic acid (EPA) seem to protect against the development of CRC, while medium-chain fatty acids (MCFA) and arachidonic acid (AA) may be associated to greater risk. Long-chain saturated fatty acids (LCSFA) do not seem to be related to this risk, whereas associations of the tumor with monounsaturated fatty acids (MUFA), trans fatty acids and polyunsatured fatty acids (PUFA) such as linoleic acid (LA), alpha-linoleic acid (ALA), docosahexaenoic acid (DHA), and the omega-3/omega-6 ratio do not seem convincing. Thus, substituting foods with high MCFA and AA content for diets rich in SCFA and EPA is suggested for reducing this risk.
Western diets are deficient in omega-3 fatty acids, in which the n-6/n-3 ratio between fatty acids varies from 15:1 to 16.7:112. This distortion is implicated in the pathogenesis of cardiovascular diseases and cancer, as well as inflammatory and immunological diseases, with the recognition that the modulation, of this ratio to 2.5:1 reduces rectal cellular proliferation in cancer patients45. However, little is yet known on the therapeutic doses of omega 3 fatty acids. It is believed that these doses depend on the severity of the illness resulting from genetic predisposition.
Calories, excess weight and smoking
The excessive consumption of calories is strongly related to the high indices of colon cancer in western countries. The implicated mechanisms in this relation ar the increases intestinal transit time, concentrations of fecal bile acids, epithelial proliferation, hyperinsulinemia and the development of resistance to insulin. Diverse studies have assessed the relation between saccharose ingestion and CRC, and 15 epidemiological studies (among 17) identified a positive association; one found a null relation8.
There is evidence that in industrialized nations obese individuals have a relatively higher risk for CRC. This phenomenon has been explained by the chronic hyperinsulinemia theory. This alteration is generated by caloric diets with western characteristics (saturated fats and refined carbohydrates), leading to a peripheral resistance to insulin. A high concentration of circulating serum insulin may have a mitogenic action on colonocytes, cellular proliferation and apoptosis22. Furthermore, it may increase the availability of insulin-like 1 growth factor (IGF-1) that promotes cellular proliferation.
Lack of physical activity, excess body weight and central deposition of adiposities have been also considered consistent risk factors. In a national cohort study14 uniting 13,420 individuals, 222 incidental cases of CRC were diagnosed. Assessment of the risk increased progressively with excess weight estimated by the body mass index (BMI), where the risk varied from 1.79 for a BMI between 22 and 24 to 2,79 for a BMI above 30. From these data, the institution of regular physical exercise can be indirectly recommended as a preventative behavioral measure against CRC.
Although smoking has often been associated to the development of colorectal adenomas, its relation with CRC has only been mentioned in recent years. Giovanucci and cols.22 suggested that smoking acts as an initiating agent for CRC, increasing the risk of adenomas after 20 years and cancer after 35 years of the addiction. This risk is greater in the right colon44.
Chemical prevention
Observation studies on individuals at risk have suggested that the use of certain supplements and drugs (non-steroid anti-inflammatory drugs, post-menopause hormonal reposition therapy, folic acid and calcium) can prevent the development of CRC18,47. However, no evidence is strong enough to recommend their routine utilization. Although anti-oxidants are hypothethically cancer preventive agents, a controlled randomized study on anti-oxidant vitamins (beta carotene, vitamin C, vitamin E) demonstrated no effects on the incidence of CRC23.
In one observational study, the use of folic acid supplements for more than 15 years determined a 75% reduction in the relative risk of CRC21. Since folate participates for DNA synthesis, its deficiency may cause abnormalities in its synthesis and repair mechanisms. The combination of an early initiation of smoking habits, high consumption of alcohol (which is an antagonist of the methyl group) and a low availability of folates generate hypomethylation of the DNA that is recognized as an early event in colorectal carcinogenesis3,22.
Similarly, it is suggestd that the link between calcium and bile acids in the intestinal lumen can inhibit carcinogenic effects. A randomized controlled study4 on the daily supplementation of 1,200 mg of elementary calcium over 4 years demonstrated a 19% reduction in the risk of recurring adenomas in a presumably medium-risk population. However, it is not yet known whether this finding applies to individuals at high risk as determined by genetic alterations9.
Among 23 epidemiological studies on calcium intake, 17 found an inverse association, nine of which with statistical significance. In regards to vitamin D, 10 of 12 studies demonstrated an inverse relation, five of which with statistical difference6.
These findings raise the idea that there may be other reasons for consuming drugs such as aspirin and folic acid (to prevent cardiovascular diseases) or ingesting calcium and strogen (to prevent osteoporosis). However, the consumption of agents with chemical prevention properties can present potential adverse effects. Thus, cautious consideration should be taken concerning the risk/benefit relation before general recommendations are made. In the case of anti-inflammatory drugs, the risk of digestive bleeding and crebral hemorrhages should be weighed against their possible benefits. More recently, studies have been developed with less toxic drugs such as sulindac and celecoxib (cyclo-oxigenase inhibitors) to investigate their effectiveness and adverse effects22.
Finally, recent meta-analysis studies have demonstrated that vitamin E (alpha-tocopherol) may have more consistent protective effects, while the inverse associations between vitamin C and beta-carotene with CRC are yet considered weak30,51.
Broad-based prevention
The data presented in this review show that diverse dietary components and behavioral characteristics may be associated to a higher risk of CRC, with variable levels of consistency. So, after identifying the risk factors (environmental or genetic) involved with CRC, primary prevention should be accomplished with modifications in lifestyle, habits and diet composition, such as:
Reduction in fat ingestion to 30% of the total calories.
Consumption of fiber in quantities of 20 to 30 grams per day.
Inclusion of a large variety of fruit and vegetables in the diet.
Limitation of alcohol consumption.
Breaking smoking habits.
Controlling obesity (reduce calories from all sources).
Practicing regular physical exercise.
Evidence of the effectiveness of primary prevention by way of dietary measures is as yet questionable51. Nevertheless, the observation that a large number of dietary and behavioral factors associated to the risk of CRC are similar to those of cardiovascular diseases and other tumor emphasizes the possibility that these modifications can bring additional benefits to people's the quality of life22.
Secondary prevention also has a critical role in reducing CRC mortality. It involves identification of risk groups (screening), treatment and follow-up of patients with pre-malignant lesions. The Brazilian Society of Coloproctology recommends the following program: (for more information visit www.combateaocancer.org.br or www.sbcp.org.br):
Proctological examination after the age of 40 or 50 years (depending on the risk group);
fecal occult bllod test after the age of 50 years;
flexible sigmoidoscopy every 3 to 5 years after the age of 50 years; abnormal findings require colonoscopy.
Recent developments in molecular techniques may have some potential to identify high risk populations for CRC by selecting individuals that should be screened, and thus improve the cost-benefit relation of these efforts. In the future, one expects to modulate the risk of CRC by dietary changes that could influence its carcinogenesis mechanisms.
References
1. Alabaster O, Tang ZC, Frost A, Shivapurkar N: Potential synergism between wheat bran and psyllium: enhanced inhibition of colon cancer. Cancer Lett 1993, 75:53-8. [ Links ]
2. Alberts DS, Martínez ME, Roe DJ, Guillén-Rodríguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, Earnest DL, Sampliner RE: Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas: Phoenix Colon Cancer Prevention Physicians'. Network N Engl J Med 2000, 342:1156-62. [ Links ]
3. Bagnardi V, Blangiardo M, La Vecchia C, Corrao G: A metaanalysis of alcohol drinking and cancer risk. Brit J Cancer 2001, 85:1700-1705. [ Links ]
4. Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER: Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999, 340(2):101-7. [ Links ]
5. Benito E, Cabeza E, Moreno V, Obrador A, Bosch FX: Diet and colorectal adenomas: a case-control study in Majorca. Int J Cancer 1993, 55:213-9. [ Links ]
6. Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjonneland A, Overvad K, Martínez C, Dorronsoro M, González CA, Key TJ, Trichopoulou A, Naska A, Vineis P, Tumino R, Krogh V, Bueno-de-Mesquita HB, Peeters PH, Berglund G, Hallmans G, Lund E, Skeie G, Kaaks R, Riboli E: Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003, 361(9368):1496501. [ Links ]
7. Bostick RM: Human studies of calcium supplementation and colorectal epithelial cell proliferation. Cancer Epidemiol Biomarkers Prev 1997, 6(11):971-80 [ Links ]
8. Bostick RM: Cancer & Nutrition: Prevention and Treatment. Nestlé Nutrition Workshop Series Clinical and Performance Program 2000, 4:67-86. [ Links ]
9. Boyapati SM, Bostick RM, McGlynn KA, Fina MF, Roufail WM, Geisinger KR, Wargovich M, Coker A, Hebert JR: Calcium, vitamin D, and risk for colorectal adenoma: dependency on vitamin D receptor BsmI polymorphism and nonsteroidal anti-inflammatory drug use? Cancer Epidemiol Biomarkers 2003, 12(7):631-7. [ Links ]
10. Burkitt DP: Related disease-related cause? Lancet 1969, 2:1229-31. [ Links ]
11. Campos FG, Waitzberg DL, Habr-Gama A, Plopper C, Terra RM: Ácidos Graxos de Cadeia Curta e Doenças Colo-Retais. Rev Bras Nutr Clin 1998, 13: 276-85. [ Links ]
12. Campos FG, Waitzberg DL, Habr-Gama A, Logullo AF, Noronha IL, Jancar S, Torrinhas RS, Furst P: Impact of parenteral n-3 fatty acids on experimental acute colitis. Br J Nutr 2002, 87(1):S83-8. [ Links ]
13. Coppini LZ, Waitzberg DL, Campos FG, Habr-Gama A: Fibras Alimentares e ácidos graxos de cadeia curta. In: Dan L. Waitzberg (ed.), Nutrição oral, enteral e parenteral na prática Clínica, São Paulo. Editora Atheneu, 2000, p. 79-94. [ Links ]
14. Ford ES: Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol 1999, 150(4):390-8. [ Links ]
15. Friedenreich CM, Brant RF, Riboli E: Influence of methodologic factors in a pooled analysis of 13 case-control studies of colorectal cancer and dietary fiber. Epidemiology 1994, 5:66-79. [ Links ]
16. Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stmapfer MJ, Rosner B, Speizer FE, Willett WC: Dietary fiber and the risk colorectal cancer and adenoma in women. N Engl J Med 1999, 340(3):169-76. [ Links ]
17. Gaard M, Tretli S, Loken EB: Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev 1996, 5(6):445-54. [ Links ]
18. Gambacciani M, Monteleone P, Sacco A, Genazzani AR: Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract Res Clin Endocrinol Metab 2003, 17(1):139-47. [ Links ]
19. Galloway DJ, Owen RW, Jarrett F, Boyle P, Hill MJ, George WD: Experimental colorectal cancer: the relationship of diet and faecal bile acid concentration to tumour induction. Br J Surg 1986, 73(3):233-7. [ Links ]
20. Giacosa A, Hill MJ, Davies GJ: Fibres and colorectal cancer: should we change our dietary advice on prevention? Dig Liver Dis 2002, 34(2):S121-3. [ Links ]
21. Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC: Multivitamin use, folate, and colon cancer in women in the Nurse's Health Study. Ann Intern Med 1998, 129(7):517-24. [ Links ]
22. Giovannucci E: Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002, 31(4):925-43. [ Links ]
23. Greenberg ER, Baron JA, Tosteson TD, Freeman DH Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW: A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med 1994, 331(4):141-7. [ Links ]
24. Hardman WE, Cameron IL: Site specific reduction of colon cancer incidence, without a concomitant reduction in cryptal cell proliferation, in 1,2-dimethylhydrazine treated rats by diets containing 10% pectin with 5% or 20% corn oil. Carcinogenesis 1995, 16:1425-31. [ Links ]
25. Hill MJ: Bacterial fermentation of complex carbohydrate in the human colon. Eur J Cancer Prev, 1995, 4(5):353-8. [ Links ]
26. Howe GR, Benito E, Castelleto R, Cornee J, Esteve J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA: Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 1992, 84(24):1887-96. [ Links ]
27. Jacobs DR, Pereira MA, Meyer KA, Kushi LH: Fiber from whole grains, but not refined grains, is inversely associated with all-cause mortality in older women: the Iowa women's health study. J Am Coll Nutr 2000, 19(3):326S-330S. [ Links ]
28. Kritchevsky D: Protective role of wheat bran fiber: preclinical data. Am J Med 1999, 106:28S-31S. [ Links ]
29. Kushi L, Giovannucci E: Dietary Fat and Cancer. Am J Med 2002, 113:63S-70S. [ Links ]
30. Longnecker MP, Martín-Moreno JM, Knekt P: Serum a=tocopherol concentration in relation to subquent colorectal cancer: data from five cohort studies. J Natl Cancer Inst 1992, 84:430-5. [ Links ]
31. MacLennan R, Macrae F, Bain C: Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas: the Australian Polyp Prevention Project. J Natl Cancer Inst 1995, 87:1760-6. [ Links ]
32. McKeown-Eyssen GE, Bright-See E, Bruce WR: A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps: Toronto Polyp Prevention Group. J Clin Epidemiol 1994, 47:525-36. [ Links ]
33. Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S: Diet and Colorectal Adenoma in Japanese Males and Females. Dis Colon Rectum 2001, 44:105-111. [ Links ]
34. National Health and Medical Research Council: The prevention, early detection, and management of colorectal cancer. Canberra: National Health and Medical Research Council. [ Links ]
35. Nordgaard I, Hove H, Clausen MR, Mortensen PB: Colonic production of butyrate in patients with previous colonic cancer during long-term treatment with dietary fibre (Plantago ovata seeds). Scand J Gastroenterol 1996, 31(10):1011-20. [ Links ]
36. Nkondjock A, Shatenstein B, Maisonneuve P, Ghadirian P: Specific fatty acids and human colorectal cancer: an overview. Cancer Detect Prev 2003, 27(1):55-66. [ Links ]
37. Peters U, Sinha R, Chatterjee N: Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet 2003, 361:1491-95. [ Links ]
38. Platz EA, Giovannucci E, Rimm EB, Rockett HR, Stampfer MJ, Colditz GA, Willett WC: Dietary fiber and distal colorectal adenoma in men. Cancer Epidemiol Biomarkers Prev 1997, 6(9):661-70. [ Links ]
39. Reddy BS, Sharma C, Simi B, Engle A, Laakso K, Puska P, Korpela R: Metabolic epidemiology of colon cancer: effect of dietary fiber on fecal mutagens and bile acids in healthy subjects. Cancer Res 1987, 47(2):644-8. [ Links ]
40. Sandhu MS, White IR, McPherson K: Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidem, Biom Prev 2001, 10:439-446. [ Links ]
41. Schatzkin A, Lanza E, Corle D: Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N Engl J Med 2000, 342:1149-55. [ Links ]
42. Sengupta S, Tjandra JJ, Gibson Pr: Dietary Fiber and Colorectal Neoplasia. Dis Colon Rectum 2001, 44:1016-1033. [ Links ]
43. Shankar S, Lanza E: Dietary fiber and cancer prevention. Hematol Oncol Clin North Am 1991, 5(1):25-41. [ Links ]
44. Sharpe CR, Siemiatycki JA, Rachet BP: The effects of smoking on the risk of colorectal cancer. Dis Colon Rectum 2002, 45:1041-1050. [ Links ]
45. Simopoulos AP: The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002, 56(8):365-79. [ Links ]
46. Sweeney C, Coles BF, Nowell S, Lang NP, Kadlubar FF: Novel markers of susceptibility to carcinogens in diet: associations with colorectal cancer. Toxicology 2002, 181-182:83-7. [ Links ]
47. Tomeo CA, Colditz GA, Willett WC: Harvard Report on Cancer Prevention. Volume 3: prevention of colon cancer in the United States. Cancer Causes Control 1999, 10(3):167-80. [ Links ]
48. Trock B, Lanza E, Greewald P: Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst 1990, 82:650-61. [ Links ]
49. La Vecchia C, Tavani A: Fruit and vegetables, and human cancer. Eur J Cancer Prev 1998, 7(1):3-8. [ Links ]
50. Waitzberg DL, Waitzberg AFL: Fibra alimentar e câncer dos cólons. Monografía ISBN 85-88984-02-4. Edição particular. São Paulo, Brasil, 2002. [ Links ]
51. Wilmink AB: Overview of the epidemiology of colorectal cancer. Dis Colon Rectum 1997, 40(4):483-93. [ Links ]












 Curriculum ScienTI
Curriculum ScienTI