Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.20 no.1 Madrid ene./feb. 2005
Original
Current use of parenteral nutrition in a pediatric hospital. Comparison to the
practise 8 years ago
J. M. Moreno Villares, F. Fernández Carrión*, J. I. Sánchez Díaz*, P. Gomis Muñoz** and M. León Sanz
Nutrition Unit. *Pediatric Intensive Carne Unit. **Pharmacy Department. Hospital Universitario 12 de Octubre. Madrid. Spain.
| Abstract Parenteral nutrition (PN) has become a mainstay in the treatment of critically ill children, and in the management of extremely premature newborns. We analyse the changes in the profile of pediatric PN in our institution during the last decade. (Nutr Hosp 2005, 20:46-51) Key words: Parenteral nutrition, children, neonates, nutrition support, liver dysfunction, catheter.
| USO ACTUAL DE LA NUTRICIÓN PARENTERAL EN UN HOSPITAL PEDIÁTRICO. COMPARACIÓN CON LA PRÁCTICA HACE 8 AÑOS Resumen La nutrición parenteral (NP) constituye un elemento fundamental en el tratamiento de los niños gravemente enfermos, así como en el cuidado de recién nacidos de muy bajo peso. Presentamos los resultados del uso de la NP en un hospital pediátrico terciario y su variación respecto a la práctica ocho años antes. (Nutr Hosp 2005, 20:46-51) Palabras clave: Nutrición parenteral, niños, neonatos, soporte nutricional, catéter, complicaciones. |
Correspondencia: José Manuel Moreno Villares
Nutrition Unit
Hospital Universitario 12 de Octubre
Ctra. de Andalucía, km 5,400
28041 Madrid
E-mail: jmoreno.hdoc@salud.madrid.org
Recibido: 20-IV-2004.
Aceptado: 21-V-2004.
The development of the technique of parenteral nutrition (PN) was closely related to its use in children, to the point that the initial report was referred to an infant1. Since then, PN has become a mainstay in the treatment of critically ill or surgical pediatric patients, as well as in the management of extremely premature newborns2.
Since the early 1990s a large number of published articles emphasized the potential danger of PN and advocated the use of enteral nutrition (EN)3,4. Based on these references it would seem that the use of PN would be decreasing and, obviously, increasing EN use. In patients with a functioning gastrointestinal system, nutritional support should preferably be based on enteral feeds, either alone or in combination with PN. Furthermore, despite recent advances in PN solutions and technique, there is still significant morbidity associated with PN therapy in childhood.
Within this scenario, our rationale was to analyse the changes in the profile of pediatric PN in a tertiary level hospital (200 pediatric/neonatal beds) during the last decade. Although we understand that the period between the two analysed years is not so long, we consider the results may help to better understand the prescription and use of PN in order to establish a plan to improve the quality of inpatient pediatric nutritional support. Our conclusions may not be representative of the general practise of PN in Spain, mainly if we realized, as reported, the great variability in prescription and elaboration of PN in different Spanish hospitals5.
Methods
The clinical records of all patients under 16 who received PN in the hospital in 1994 and 2002 were reviewed. Hospital 12 de Octubre is a tertiary level hospital with 200 pediatric beds, including 14 Pediatric Intensive Care Unit beds and 50 Neonatal Care Unit beds. The Nutrition Support team was establish in 1992 and directly supervises all the non-neonatal PN orders. Table I shows the number of patients who received PN as well as the number of bags elaborated.
Definitions
A patient was considered newborn if, at the time of the beginning of PN, his/her age was under 28 days.
There are three possibilities of prescription in our institution: tailored solutions, standardized solutions, and partially standardized (tailored energy and protein needs plus standard electrolytes, vitamins and minerals)6.
The definitions of catheter related infection closely follow those of the Centres for Disease Control Guidelines7.
We consider PN-related liver disease when conjugated bilirubin was > 2.0 g/dL and/or GGT, GOT (AST) or GPT (ALT) were higher x2 normal values in our laboratory in two consecutive samples in the absence of previous liver disease.
Epidemiological data as well as the composition of the solutions were recorded. Student t test and Chisquare were used for comparisons as appropriate. P value < 0.05 was considered as statistically significant.
Results
The frequency of use did not change during the period of study, neither in the total number nor if related to the percentage of hospital admissions (table II). The apparent decrease in relative use in neonates is explained by an administrative change: while in 1994 only the admissions in the Neonatal Intensive Care Unit (NICU) were registered, in 2002 all neonates admitted to the Neonatal Unit were taken into account. Most of the patients, both neonates and children, were located in the NICU and the Pediatric Intensive Care Unit (PICU) when the PN was first started.
In 2002 the number of pediatric patients who received PN because of a non-surgical gastrointestinal disease (e.g. Crohn's disease) decreased. In neonates prematurity as the main indication of PN increased from 1994 to 2002 (fig. 1). Mean length decreased in 2002, although with no significant difference (children: 11 days, SD 14.9 in 2002 vs 15.2 (SD 14.8) in 1994; neonates, 9.2 (SD 8.2) in 2002 vs 10 (SD 8.7) days in 1994).
Neonatal PN was always tailored as per protocol in our institution, whilst near 2/3 of PN are partially or totally standardized in children. Although we could only record the data from 2002, it took 1.5 days (SD: 0.96) in children to achieve the estimated requeriments against 3.75 (SD: 2.39) in neonates. The composition of the PN in the first day is shown in table III.
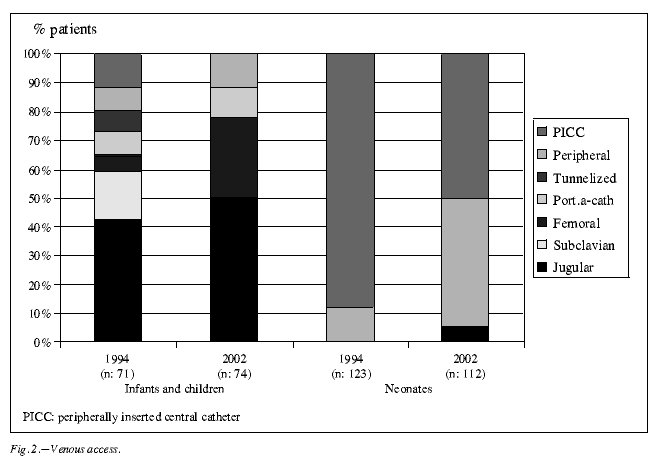
The type of intravenous access has changed over the years. In infants and children the subclavian vein was no longer used, while the femoral vein has become the 2nd venous access after the internal jugular vein. The jugular vein is the preferable access used by the anesthesiologists in the operating room; the femoral vein by the intensive care unit pediatricians. In neonates, peripheral venous cannulae have been increasingly used (fig. 2).
The main reason to stop PN was the change to the oral/enteral route in both neonates (91% in 1994) and children (79% in 1994; 86,8% in 2002, p < 0.05).
Because of the retrospective method of the study we could only analyze the complications in the group of infants and children. There is a trend to a lower frequency of complications with no statistical difference (table IV).
Discussion
Parenteral nutrition has a critical role in the treatment of neonates and children with intestinal failure as well as extreme prematurity, critical illness, acute pancreatitis or malignancies8. Despite recent advances, there is still significant morbidity asociated with PN in childhood; therefore patients receiving this therapy need to be closely monitored for evidence of metabolic disturbance, sepsis, catheter-related complications and micronutrient deficiencies or excesses9. The implication of Nutrition Support Teams in the care of PN patients has improved clinical and economical outcomes by decreasing the incidence of complications, optimal selection of the feeding route, and by reducing wastage of PN solutions and resources10. Nevertheless periodic studies on the quality of PN need to be done in each institution in order to analyse causes and factors related to detect defects and look for solutions11,12.
In this study we analysed the changes in PN support for pediatric patients in our department. The results showed that the number of patients, underlying diseases, and indications did not significatively change over the period of study. The percentage of neonates increased because of the increase in the number of extreme premature babies partly due to the development of assisted reproductive technology13.
PN composition is thought to cover all the requeriments of energy and nutrients. Over the years we have estimated the Basal Metabolic Rate of the majority of pediatric patients according to the Schofield equations14 multiplying by a factor of 1.2-1.515-17. The maintenance requeriments for water are determined by the caloric expenditure. By means of the classic HollidaySegar formula it is possible to determine the water requeriments from weight alone18.
Maintenance requirements of sodium, chloride, and potassium are 3.0, 2.0, and 2.0 mEq/100 kcal/day, repectively. This allows us to elaborate a PN solution with standardized electrolytes adjusted to volume. Standardization of PN simplyfies elaboration and decreases the risk of mistakes during the process; on the contrary, customized PN allows flexibility and individualized solutions19. We have chosen a system that makes possible to have both standard and tailored solutions7. Almost 2/3 of our non-neonatal patients use totally or partially standardized solutions.
The timing of PN progression is also under discussion. In 2002 it took 1.5 days in infants and children and 3.75 days in neonates to achieve the total estimated requeriments. As a result of the aggressive enteral support in neonates, the length of PN has shortenned20.
Despite the development and commercial availability of new solutions, the nutritional components have remained almost identical during the period of study. Glucose constitutes the main energy source, but avoiding to exceed the maximum rate of glucose oxidation21. We use intravenous fat emulsions daily not only for the prevention of essential fatty acid deficiency but also as an energy source. In 1994, we used traditional soy bean-based solutions (Intralipid 20%®, Fresenius Kabi) in neonates and MCT/LCT emulsions in infants and children (Lipofundin®, Braun) but recently we have switched to an olive oil-based lipid emulsion (ClinOleic®, Baxter Healthcare corporation). No problems in the tolerance to intravenous fat emulsions or in the rate of complications have been observed with the change of type of lipid emulsion. Carnitine is not routinely added to our PN mixture.
Our policy is to use 3-in-1 admixture of nutrient solution whenever possible. Nevertheless, in neonates the use of "all-in-one" admixture has been exceptional because the risk of destabilization. Several reports have described the incompatibility of all-in-one PN admixtures and heparine22,23 and in consequence our guidelines do not include heparine in the mixtures. If the risk of catheter thrombosis outweight the advantages of the "3-in-1" admixture, e.g. very low infusion rates, where the use of heparine may be advisable, we choose not to use "3-in-1" mixtures.
Amino acid requirements vary from ≥ 2.5 g·kg-1·d-1 for preterm infants to 0.75 g·kg-1·d-1 for adolescents24. It has been stated in the literature that amino acid intakes should be gradually increased over the first few days of PN administration. We consider, in agreement with Shulman and Phillips17, that there is no good scientific support for this view. Our practice is to start with the total requeriments in the first day of PN and we have not observed any negative metabolic consequences. Our practice in neonates has moved from the more conservative approach to early amino acid introduction25,26. The pediatric amino acid solutions used in our instituon contain essential amino acids, including branched chain amino acids, adapted to the special needs of the neonate (Trophamine®, McGraw Hill in 1994; Primene®, Baxter Healthcare corporation in 2002). These solutions are employed in patients up to 10 years old.
Vitamin and mineral supplementation follows the guideline of the American Society for Clinical Nutrition27, with some modifications for the prematures. Adult parenteral vitamin and mineral solutions are used for children older than 10 years. We slightly modify the supplementation in case of liver or renal disease, although there are no individual preparations for intrevenous minerals in Spain except for zinc and iron.
Regarding the intravenous access, the most significant change along the period of study has been the decreased use of the subclavian vein in infants and children. Its room has been occupied by the femoral vein. Internal jugular vein continues being the most current access. In our series the use of tunnelized devices for the infusion has almost dissapeared. The explanation is that for bone marrow infusion in bone marrow transplant patients a tunnelized venous access was required in 1994, while in 2002 is a percutaneous one. Home parenteral nutrition patients are not considered in this review. Because of the use of an early aggressive enteral support in premature infants, peripheral venous cannulae have had a steadily increasing role as intrevenous access for PN in neonates although peripheral percutaneous intravenous central catheters are still the most common access.
Similarly to other reports12, the indications for PN have not changed over time. Prematurity is the first indication in neonates. In older children postoperative care secondary to gastrointestinal diseases or congenital malformations was the most common indication.
We could only record the frequency of complications in the non-neonatal group. Liver disfunction was the most common complication, although mild and transient. It was not necessary to stop PN because of this reason in any case. The existence of a Nutrition Support Team and the practices to avoid complications of central venous cathetherization28 may explain the low frequency of infectious complications.
PN is still a life-saving therapy as it has been for over 40 years. Despite the increasing use of EN, the frequency of use and indications has not shown significant differences over time in our experience. Adopting a more pro-enteral nutrition approach will enable children to be fed optimally with fewer complications.
References
1. Wilmore DW, Dudrick SJ: Growth and development of an infant receiving all nutrients exclusively via a vein. JAMA 1968, 20:860-864. [ Links ]
2. Schewnk WF: Specialized nutrition support: the pediatric perspective. J Parent Ent Nutr 2003, 27:160-167. [ Links ]
3. AGA Technical review on parenteral nutrition. Gastroenterology 2001, 121:970-1001. [ Links ]
4. Collier S, Lo C: Advances in parenteral nutrition. Curr Opinion Pediatr 1996, 8:476-482. [ Links ]
5. Moreno Villares JM, Fernández-Shaw Toda C, Muñoz García MJ, Gomis Muñoz P: Encuesta sobre la variabilidad en la elaboración de la nutrición parenteral en pediatría. Nutr Hosp 2002, 17:251-255. [ Links ]
6. Moreno Villares JM, Fernández-Shaw C, Gomis Muñoz P, Valero Zanuy MA, León Sanz M: Nutrición parenteral en pediatría: ¿soluciones normalizadas mejor que individualizadas? An Esp Pediatr 2002, 57:29-33. [ Links ]
7. Hodge D, Puntis JW: Diagnosis, prevention and management of catheter related bloodstream infection during long-term parenteral nutrition. Arch Dis Child Fetal Neonatal Ed 2002, 87:F21-F24. [ Links ]
8. Heine RG, Bines JE: New approaches to parenteral nutrition in infants and children. J. Paedriatr Child Health 2002, 38:433-437. [ Links ]
9. Meadows N: Monitoring and complications of parenteral nutrition. Nutrition 1998, 14:806-808. [ Links ]
10. Newton R, Timmis L, Bowling TE: Changes in parenteral nutrition supply when the nutrition support team controls prescribing. Nutrition 2001, 17:347-348. [ Links ]
11. Gómez-Ramos MJ, Saturno Hernández PJ: Utilización de la nutrición parenteral total en un hospital general: criterios de calidad y factores asociados a su cumplimiento. Med Clin (Barc) 2002, 119:686-689. [ Links ]
12. Suita S, Yamanouchi T, Masumoto K, Ogita K, Nakamura M, Taguchi S: Changing profile of parenteral nutrition in pediatric surgery: a 30-year experience at one institution. Surgery 2002, 131:S275-S282. [ Links ]
13. Kovalewsky G, Coutifaris C: Low and very low birth weight after use of assisted reproductive technology. N Engl J Med 2002, 347:1451. [ Links ]
14. Schofied W: Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985, 39C S1:5-41. [ Links ]
15. Sentengo TA, Tershakovec AM, Mascarenhas MR, Watson MH, Stallings VA: Resting energy expenditure and prediction equations in young children with failure to thrive. J Pediatr 2000, 136:345-350. [ Links ]
16. Chwals WJ, Lally KP, Woolley MM, Mahour GH: Measured energy expenditure in critically ill infants and young children. J Surg Res 1988, 44:467-472. [ Links ]
17. Shulman RJ, Phillips S: Parenteral nutrition in infants and children. J Pediatr Gastroenterol Nutr 2003, 36:587-607. [ Links ]
18. Holliday MA, Segar WE: The maintenance need for water in parenteral fluid therapy. Pediatrics 1957, 19:823-832. [ Links ]
19. Stettler N, Sentongo TA, Carroll M, Schears GJ, Mascarenhas MR: Impact of customized parenteral nutrition in a pediatric hospital. NCP 2001, 16:345-348. [ Links ]
20. Wilson DC, Cairns P, Halliday HL, Reid M, McClure G, Dodge JA: Randomized controlled trial of an agressive nutritional regimen in sick very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1997, 77:F4-11. [ Links ]
21. Jones MO, Pierro A, Hammond P, Nunn A, Lloyd DA: Glucose utilization in the surgical newborn infant receiving total parenteral nutrition. J Pediatr Surg 1993, 1121-1125. [ Links ]
22. Johnson OL, Washington C, Davis SS, Bentley PK, Lowe KC: The destabilization of parenteral feeding emulsions by heparin. Int J Pharm 1989, 53:237-240. [ Links ]
23. Silvers KM, Darlow BA, Winterbourn CC: Pharmacologic levels of heparin do not destabilize neonatal parenteral nutrition. J Parent Ent Nutr 1998, 22:311-314. [ Links ]
24. National Advisory Group on Standards and Practice Guidelines for Parenteral Nutrition. Safe practices for parenteral nutrition formulations. J Parent Ent Nutr 1998, 22:49-66. [ Links ]
25. Thureen PJ, Melara D, Fennessey PV, Hay WJ: Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res 2003, 53:24-32. [ Links ]
26. Porcelli PJ, Sisk PM: Increased parenteral amino acid administration to extremely low-birth-weight infants during early postnatal life. J Pediatr Gastroenterol Nutr 2002, 34:174179. [ Links ]
27. Greene HL, Hambridge KM, Schanler R, Tsang RC: Guidelines for the use of vitamins, trace elements, calcium, magnesium and phosphorus in infants and children receiving total parenteral nutrition: report of the committee on clinical practice issues of the American Society for Clinical Nutritional. Am J Clin Nutr 1988, 48:1324-1342. [ Links ]
28. McGee DC, Gould MK: Preventing complications of central venous catheterization. N Engl J Med 2003, 348:1123-1133. [ Links ]