Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.5 Madrid sep./oct. 2013
https://dx.doi.org/10.3305/nh.2013.28.5.6505
ORIGINAL / Alimentos funcionales
Green juice as a protector against reactive species in rats
Zumo verde como protector frente a las especies reactivas en ratas
Pathise S. Oliveira1, Tatiana D. Saccon1, Tatiane M. da Silva1, Marcelo Z. Costa3, Filipe S. P. Dutra1, Alana de Vasconcelos1, Claiton L. Lencina2, Francieli M. Stefanello3 and Alethéa G. Barschak3,4
1Universidade Federal de Pelotas. Campus Universitario. Pelotas. RS. Brazil
2Centro de Ciências Químicas, Farmacêuticas e de Alimentos. Curso de Farmacia. Universidade Federal de Pelotas. Pelotas. RS. Brazil
3Programa de Pós-Graduação em Bioquímica e Bioprospecção. Universidade Federal de Pelotas. Pelotas. RS. Brazil
4Departamento de Ciencias Básicas da Saúde. Universidade Federal de Ciéncias da Saúde de Porto Alegre. Porto Alegre. RS. Brazil
This work was supported in part by grants from "Fundaçâo de Amparo à Pesquisa do Rio Grande do Sul" (FAPERGS), "Coordenaçâo de Aperfeiçoamento de Pessoal de Nivel Superior" (CAPES) and "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq), Brazil.
ABSTRACT
Introduction: Green juice is popularly known for introducing antioxidants, improving intestinal function and reducing weight gain.
Objectives: In the present study we determine the antioxidant effect of green juice comparing it with orange juice.
Methods: Rats were divided into three experimental groups and submitted to supplementation for 15 days: the (GJ) group received green juice, the (OJ) group received orange juice and the control group received water. We evaluated the antioxidant activity and total phenolic content of green and orange juices, as well as rat weight gain. We also investigated some oxidative stress parameters, namely thiobarbituric acid-reactive substances (TBARS), superoxide dismutase and catalase in rat cerebral cortex.
Results and discussion: Results showed that GJ had significantly less weight gain than the control group. With respect to antioxidant activity screening, the remaining percentage of DPPH at dilutions 1:10, 1:100 and 1:1000 of green juice was 22.8%, 58% and 78%, and orange juice, at the same dilutions, was 5.6%, 5.6% and 77.2%, respectively. The ability of juices to reduce the ABTS radical was 3.5 mmol trolox/L for green juice and 5.2 mmol trolox/L for orange juice. Additionally, the green juice did not present any difference in total phenolic acid content when compared to orange juice. TBARS were reduced in GJ and OJ. Besides, GJ supplementation decreased catalase activity. In conclusion, our data showed that green juice reduced weight gain, lipoperoxidation and catalase activity, suggesting that this supplementation may have a protective effect against reactive species.
Key words: Green juice. Oxidative stress. Antioxidant. Weight gain. Rats.
RESUMEN
Introducción: El zumo verde es conocido popularmente como fuente de antioxidantes, mejorando la función intestinal y reduciendo la ganancia de peso.
Objetivos: En este estudio determinamos el efecto antioxidante del zumo verde en comparación con el zumo de naranja.
Métodos: Se dividió a las ratas en tres grupos experimentales y se las sometió a un suplemento durante 15 días: el grupo ZV recibió zumo verde, el grupo ZN recibió zumo de naranja y el grupo control recibió agua. Evaluamos la actividad antioxidante y el contenido total en fenoles de los zumos verde y de naranja, así como la ganancia de peso en las ratas. También investigamos algunos parámetros del estrés oxidativo, en concreto las sustancias reactivas del ácido tiobarbitúrico (SRATB), la superóxido dismutasa y la catalasa en la corteza cerebral de las ratas.
Resultados y discusión: Los resultados mostraron que el ZV producía una ganancia de peso significativamente menor que en el grupo control. Con respecto al estudio de la actividad antioxidante, el porcentaje restante de DPPH en diluciones al 1:10, 1:100 y 1:1000 de zumo verde fue del 22,8%, 58% y 78%, y para el zumo de naranja, a las mismas diluciones, fue del 5,6%, 5,6% y 77,2%, respectivamente. La capacidad de los zumos para reducir el radical de ATB fue de 3,5 mmol trolox/l para el zumo verde y de 5,2 mmol trolox/l para el zumo de naranja. Adicionalmente, el zumo verde no mostró ninguna diferencia en el contenido total de ácido fenólico en comparación con el zumo de naranja. Las SRATB se redujeron con el ZV y el ZN. Además, el suplemento con ZV disminuyó la actividad catalasa. En conclusión, nuestros datos mostraron que el zumo verde redujo la ganancia de peso, la lipo-peroxidación y la actividad catalasa, lo que sugiere que este suplemento podría tener un efecto protector frente a las especies reactivas.
Palabras clave: Zumo verde. Estrés oxisativo. Antioxidante. Ganancia de peso. Ratas.
Abbreviations
ABTS: 2,2'-azino-bis-3-ethyl benzothiazoline-6-sulfonic acid.
CAT: Catalase.
DPPH: 2,2-diphenyl-1-pycrylhydrazyl.
GJ: Green juice.
GPx: Glutathione peroxidase.
GSH: Glutathione.
OJ: Orange juice.
RS: Reactive species.
SOD: Superoxide dismutase.
TBARS: Thiobarbituric acid-reactive substances.
TEAC: Trolox equivalent antioxidant capacity.
Introduction
Reactive species (RS) such as hydrogen peroxide, superoxide, hydroxyl radical can induce damage to cellular macromolecules, including lipids, proteins and DNA. It is known that cellular redox state is a consequence of the balance between the levels of oxidizing and reducing agents and endogenous antioxidants. However, during some pathological process may occur an imbalance between oxidants and antioxidants, namely as oxidative stress.1 RS are kept at physiological levels by antioxidant defense systems, including non-enzymatic antioxidants such as glutathione (GSH), bilirubin, uric acid, vitamins C and E, and endogenous compounds with enzymatic properties such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT).1
Epidemiologic studies have demonstrated that the consumption of fruits and vegetables is inversely associated with morbidity and mortality from degenerative diseases.2 Thus, some research studies reported that antioxidants present in foods, such as vitamins C and E, selenium, polyphenols and β-carotene, may prevent some of the processes involved in the progression of cancer and cardiovascular disease.3However, the precise contribution of these dietary components to maintain health and delay disease onset is uncertain.4
Apples contain various types of polyphenols, such as phenolic acid derivatives and flavonoids, which present many physiological functions.5,6,7 A relationship has been reported between the consumption of apples or related products and chronic human diseases such as cancer and cardiovascular disease.8,9 Furthermore, fruit juice, particularly orange juice, is also an excellent source of antioxidant in diet. It is rich in bioactive compounds such as ascorbic acid, vitamin B, fiber, iron, β-carotene, flavonoids and others.10 There are many reports showing the major of orange juice in the protection of DNA against oxidative damage.11,12Besides, vitamin C seems to be essential for antioxidant homeostasis and as a cofactor in a series of post-translational events.13
Green juice contains fruits and vegetables such as apple, orange, dark green vegetables, and it is considered a great source of fibers, vitamins and minerals. This juice is popularly known for introducing antioxidants, improving intestinal function and reducing weight gain. Although there are no studies showing the effect of this juice on biological systems, it has aroused great interest among researchers because its components have different functional properties such as improving the immune system and reducing the action of RS that contribute to the progression of many diseases.14
Oxidative stress is involved in the pathogenesis of several disorders.15,16 However, in this study we evaluated the antioxidant effect of green juice to identify new dietary sources that can prevent oxidative damage.
Methods and materials
Animals
Sixty-day old male Wistar rats were obtained from the Central Animal House of Universidade Federal de Pelotas, RS, Brazil. The animals were maintained on 12 h light/12 h dark cycles at a constant room temperature (20-24o C) with free access to water and food.
Treatment
The experiments were performed after approval by the Ethics Committee on Animal Experiments (CEEA No 6303) of Universidade Federal de Pelotas and all efforts were made to minimize animal suffering. The animals were randomly divided into three experimental groups and submitted to supplementation for 15 days: group (GJ) received green juice, group (OJ) received orange juice and the control group received water. The rats were weighed at the beginning and the end of the experiment to determine the weight gain. All animals were handled for 15 days and were given 5 mL/kg orally. Animals were sacrificed 24 h after the last administration by decapitation without anesthesia; cerebral cortex was collected and was kept frozen until the biochemical analyzes.
Preparation of juice
Green juice was prepared using the following components: orange (Citrus sinensis), apple (Malus sp.), lettuce (Lactuca sativa), cabbage (Brassica oleracea) and cucumber (Cucumis sativus). Orange juice was prepared with orange (Citrus sinensis). All components were obtained commercially in Pelotas, RS, Brazil. Green juice preparation consisted of processing fruits and vegetables with water and then straining to separate the solids. Orange juice was also strained after preparation. Both green and orange juices were prepared daily.
Tissue preparation
Cerebral cortex was homogenized in sodium phosphate buffer pH 7.4 containing KCl (1:10, w/v). The homogenates were centrifuged at 3,500 rpm for 10 min at 4o C. Immediately the supernatant was separated and used for biochemical determinations.
Determination of antioxidant activity
The antioxidant activity of green juice and orange juice was measured by 2,2-diphenyl-1-pycrylhydrazyl (DPPH) radical scavenging assay according to Brand-Williams17 with some modifications. Different dilutions of the juices were prepared: A (1:10), B (1:100) e C (1:1000), which subsequently react with DPPH (60 pM). Ascorbic acid (D) was used as standard (30 pM). The decrease in absorbance was measured and the ability to scavenge free radicals was calculated based on decreased absorbance. The antioxidant activity was expressed as the percentage of DPPH remaining.
The 2,2'-azino-bis-3-ethyl benzothiazoline-6-sulfonic acid (ABTS) method is based on the ability of antioxidant molecules to quench the long-lived ABTS'+, blue-green chromophore with characteristic absorption at 734 nm, compared to trolox, a water-soluble vitamin E analog.18Results were expressed as TEAC (trolox equivalent antioxidant capacity) in mmol of trolox per L of sample.
Determination of phenolic acids
The concentration of phenolic acids was determined using the method described by Singleton and Rossi,19with modifications. The diluted sample (1:10) was added to a 10 mL volumetric with diluted Folin-Ciocalteu (1:10) Ca2CO3 20%. Next it was left in a water bath at 50o C for 5 min. Then, the readings were taken at 765 nm. The procedure was the same as the blank and with the standard solutions of gallic acid. The total phenolic content was expressed as gallic acid equivalents in milligrams per liter.
Thiobarbituric acid-reactive substances (TBARS)
TBARS, a measure of lipid peroxidation, was determined according to the method described by Ohkawa et al.20 Briefly, tissue supernatant was mixed with 20% trichloroacetic acid and 0.8% thiobarbituric acid and heated in a boiling water bath for 60 min. TBARS were determined by the absorbance at 535 nm and reported as nmol TBARS/mg protein.
Catatase (CAT) assay
CAT activity was assayed in brain cortex according to Aebi21 based on the decomposition of H2O2 monitored at 240 nm at ambient temperature. One CAT unit is defined as one pmol of hydrogen peroxide consumed per minute and the specific activity is reported as units/mg protein.
Superoxide dismutase (SOD) assay
SOD activity was assayed in cerebral cortex using the SD125-RANSOD kit of Randox. The results were expressed as units of activity of the SOD/mg of protein.
Protein determination
Protein was determined by the method of Lowry22using bovine serum albumin as standard.
Statisticat anatysis
Data were expressed as mean ± standard deviation. The comparisons of means were analyzed by Student's t test or by analysis of variance (ANOVA) followed by one-way Tukey test when the F value was significant. A value of P < 0.05 was considered to be significant. Analyses were performed using the Statistical Package for Social Sciences (SPSS).
Results
The percentage of DPPH remaining at dilutions A (1:10), B (1:100) and C (1:1000) of green juice was 22.8%, 58% and 78%, and orange juice, at the same dilutions were 5.6%, 5.6% and 77.2%, respectively. The remaining percentage of DPPH in the presence of ascorbic acid was 0.4% (fig. 1). The ability of juices to reduce ABTS radical was 3.5 mmol trolox/L for green juice and 5.2 mmol trolox/L for orange juice.
There was no significant difference in total phenolic acid content in the green juice compared to the orange juice (table I).
The TBARS, which reflects the contents of malondialdehyde, a biomarker of lipid peroxidation, was lower in the cerebral cortex of rats supplemented with green juice when compared to the control and OJ groups [F(2,27) = 38.745, P < 0.001] (fig. 2).
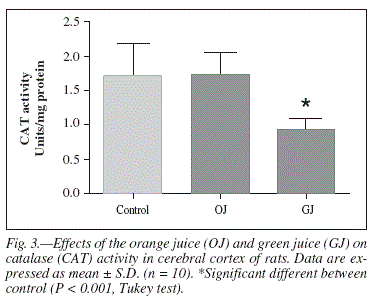
CAT activity was significantly lower in the group supplemented with green juice when compared to control [F(2,27) = 12.634, P < 0.001] (fig. 3). No significant difference was observed in SOD activity between the supplemented groups and controls, however the OJ group presented lower SOD activity than the GJ group [F(2,27) = 7.90, P < 0.05] (fig. 4).
With respect to weight gain, it was observed that the GJ group (42.2 ± 5.14 g) had significantly less weight gain than the control group (52.3 ± 6.13 g) [F(2,27) = 8.446, P < 0.001].
Discussion
It is known that fruits, vegetables and teas provide health benefits,7,23,24,25 and that some of these effects can be assigned to antioxidant compounds.24,26 Different substances in foods, especially fruits and vegetables, such as vitamins C and E, β-carotene and phenolic compounds are excellent antioxidants that are able to scavenge reactive species. However, the exact health-promoting effect of these dietary components is still unclear. Therefore, the main objective of this study was to evaluate the antioxidant effect of green juice which combines fruits and vegetables.
Phenolic compounds, ubiquitous in fruits and vegetables, represent an important group of natural antioxidants. Phenolic compounds include flavonoids and phenolic acids considered desirable bioactive foods because of their antioxidant activity.24,27 In our study, no difference was observed between the phenolic acids content of orange and green juice. A higher concentration of phenolic acids in green juice was expected because it contains apple, lettuce and other vegetables;28 however, this was not observed. It is likely that other natural antioxidants are present in both juices, thus it could be useful to identify others compounds which may contribute to antioxidant activity in these beverages.
Our results also showed that orange juice presented a better DPPH and ABTS radicals scavenging capacity than green juice. The DPPH assay is based on the ability of hydrogen-donating antioxidants to scavenge the DPPH radical.17 Antioxidants may react in different manner to distinct radical or oxidant sources, suggesting that green and orange juices may act differently in biological systems.26,29 It was observed that the smaller the dilution the greater the antioxidant activity of the juice, confirming that in natura (no dilution) juice presents more antioxidant compounds demonstrating better action against free radicals.
We have demonstrated that animals supplemented with green juice presented low levels of lipid peroxidation compared to controls and to animals supplemented with orange juice. These results may be attributed to antioxidants such as phenolic acids, vitamin C, anthocyanins and others, present in fruits and vegetables that are used to make green juice.11,24,27 Many authors reported that fruits and vegetables may provide protection against oxidative damage through antioxidants.30,31,32 Studies have demonstrated the protective effect of fruits and vegetables in different diseases including cardiovascular diseases, cancer and aging-related disorders.8,11,33 A closer look at our results allow us to suppose that other bioactive compounds in apple, lettuce, cabbage and cucumber may be responsible for the greater effect on lipid peroxidation demonstrated by green juice.
Antioxidant enzymes are an important part of the antioxidant system in aerobic organisms. The enzymatic antioxidant defenses include SOD, CAT and GPx, which help control reactive species levels.1Green juice supplementation significantly reduced catalase activity, however no change was observed in SOD activity. Catalase plays a critical role in protecting cells against toxic effects of hydrogen peroxide, catalyzing the decomposition of it and producing H2O and O2.34 It is possible that antioxidant compounds in green juice have taken over the oxidative defense and thus induced an adaptation in CAT reducing its activity.
Besides antioxidant activity, green juice also reduced weight gain in tested animals. The group supplemented with green juice presented less weight gain (20%) than the control group. Fruits and vegetables such as apple, orange, lettuce and cabbage (green juice components) are rich in soluble and insoluble fibers.35,36 Studies have shown that higher fiber diets are associated with lower body weight and adiposity.37 Fibers are hypothesized to affect body weight by delaying gastric emptying and creating a sense of fullness. Increased fiber intakes may have satiety producing effects through their effect on gut hormone production.37,38
In summary, green juice supplementation reduced weight gain, lipid peroxidation and CAT activity, suggesting that this supplement protects against reactive species reducing oxidative damage. Furthermore, green juice can be a new approach to control and maintain body weight protecting against the development of obesity. Although promising, these results should be treated with caution, because it is a single study involving animals. Further research is necessary to better understand the effects of green juice and its components before using this supplement to prevent oxidative stress or obesity.
References
1. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, fourth ed, Oxford University Press, Oxford, 2007. [ Links ]
2. Núñez-Córdoba JM, Martínez-González MA. Antioxidant vitamins and cardiovascular disease. Curr Top Med Chem 2011; 11: 1861-9. [ Links ]
3. Palafox-Carlosb H, Ayala-Zavala JF, González-Aguilar GA. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J Food Sci 2011; 76: R6-R15. [ Links ]
4. Gutteridge JM, Halliwell B. Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun 2010; 393: 561-4. [ Links ]
5. Saito T, Miyake M, Toba M, Okamatsu H, Shimizu S, Noda M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol Immunol 2002; 46: 249-55. [ Links ]
6. Shoji T, Akazome Y, Kanda T, Ikeda M. The toxicology and safety of apple polyphenol extract. Food Chem Toxicol 2004; 42: 959-67. [ Links ]
7. Quiñones M, Miguel M, Aleixandre A. Los polifenoles, compustos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr Hosp 2012; 27: 76-89. [ Links ]
8. Elberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature 2000; 405: 903-4. [ Links ]
9. Xing N, Chen Y, Mitchell SH, Young CYF. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 2001; 22: 409-14. [ Links ]
10. Stella SP, Ferrarezi AC, Santos KO, Monteiro M. Antioxidant activity of commercial ready-to-drink orange juce and nectar. J Food Sci 2011; 76: C392-C397. [ Links ]
11. Sun J, Chu Y, Wu X, Liu RH. Antioxidant and Antiproliferative Activities of Common Fruits. J Agric Food Chem 2002; 50: 7449-54. [ Links ]
12. Liu J, Dong H, Chen B, Zhao P, Liu RH. Fresh apples suppress mammary carcinogenesis, proliferative activity, and induce apoptosis in the mammary tumors of the Sprague-Dawley rat. J Agric Food Chem 2009; 57: 297-304. [ Links ]
13. Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol 2009; 157: 1097-110. [ Links ]
14. Pellegrini, N. Evaluation of antioxidant capacity of some fruit and vegetable foods: efficiency of extraction of a sequence of solvents. J Sci Food Agric 2007; 87: 103-11. [ Links ]
15. Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 2012; 110: 1109-24. [ Links ]
16. Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J Immunopathol Pharmacol 2012; 25: 345-53. [ Links ]
17. Brand-Williams W, Cuveli ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 1995; 28: 25-30. [ Links ]
18. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 1999; 26: 1231-7. [ Links ]
19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer J Enol Viticult 1965; 20: 144-58. [ Links ]
20. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-8. [ Links ]
21. Aebi H. Catalase in vitro. Methods Enzymol 1984; 105: 121-6. [ Links ]
22. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-75. [ Links ]
23. He F, Nowson C, Lucas M, Macgregor G. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Metaanalysis of cohort studies. J Hum Hypertens 2007; 21: 717-28. [ Links ]
24. Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr 2011; 2: 408-20. [ Links ]
25. Sanchez-Muniz FJ. Dietary fibre and cardiovascular health. Nutr Hosp 2012; 27: 31-45. [ Links ]
26. Gülçin I. Antioxidant activity of food constituents: an overview. Toxicol 2012; 86: 345-91. [ Links ]
27. Gui-Fang Deng, Chen Shen, Xiang-Rong Xu, Ru-Dan Kuang, Ya-Jun Guo, Li-Shan Zeng, Li-Li Gao, Xi Lin, Jie-Feng Xie, En-Qin Xia, Sha Li, Shan Wu, Feng Chen, Wen-Hua Ling and Hua-Bin Li. Potential of fruits wastes as natural resources of bioactive compounds. Int J Mol Sci 2012; 13: 8308-23. [ Links ]
28. Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S. Which poliphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem 2005; 53: 4985-9. [ Links ]
29. Huang D, Ou B, Prior RL. The Chemistry behind Antioxidant Capacity Assays. J Agric Food Chem 2005; 53: 1841-56. [ Links ]
30. Ademosun AO, Oboh G. Inhibition of acetylcholinesterase activity and Fe2+-induced lipid peroxidation in rat brain in vitro by some citrus fruit juices. J Med Food 2012; 15: 428-34. [ Links ]
31. Vieira FG, Di Pietro PF, da Silva EL, Borges GS, Nunes EC, Fett R. Improvement of serum antioxidant status in humans after the acute intake of apple juices. Nutr Res 2012; 32: 229-32. [ Links ]
32. Ferreira de Araujo PR, da silva Santos V, Rodrigues Machado A, Gevehr Fernandes C, Silva JA, da Silva Rodrigues R. Benefits of blackberry (Rubbus spp.) relative to hypercholesterolemia and lipid peroxidation. Nutr Hosp 2011; 26: 984-90. [ Links ]
33. Ganesan K, Kumar KS, Rao PVS Comparative assessment of antioxidant activity in three edible species of green seaweed, Enteromorpha from Okha, Northwest coast of India. Innov Food Sci Emerg 2011; 12: 73-8. [ Links ]
34. Goyal MM, Basak A. Human catalase: looking for complete identity. Protein Cell 2010; 1: 888-97. [ Links ]
35. Nawirska A, Kwasniewska M. Dietary fiber fractions from fruit and vegetable processing waste. Food Chem 2005; 91: 221-5. [ Links ]
36. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr 2012; 3: 506-16. [ Links ]
37. Anderson JW, Baird P, Davis Jr RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev 2009; 67: 188-205. [ Links ]
38. Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 2011; 69: 245-58. [ Links ]
![]() Correspondence:
Correspondence:
Alethéa Gatto Barschak
Universidade Federal de Ciências da Saúde de Porto Alegre
Rua Sarmento Leite, 245
CEP 90050-170 Porto Alegre. RZ. Brazil
E-mail: aletheagatto@gmail.com
Recibido: 18-I-2013
1.a Revisión: 14-II-2013
Aceptado: 18-II-2013