Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.34 no.5 Madrid sep./oct. 2017
https://dx.doi.org/10.20960/nh.1300
Probiotics for fibromyalgia: study design for a pilot double-blind, randomized controlled trial
Probióticos en fibromialgia: diseño de un estudio piloto doble ciego y randomizado
Pablo Roman1, Ángeles F. Estévez2, Nuria Sánchez-Labraca3, Fernando Cañadas2, Alonso Miras4 and Diana Cardona3
1Department of Nursing. Universitat Jaume I. Castellón, Spain.
2Department of Psychology. Universidad de Almería. Almería, Spain.
3Department of Nursing Science, Physiotherapy and Medicine. Universidad de Almería. Almería, Spain.
4Independent Researcher. Almería, Spain
ABSTRACT
Background: Fibromyalgia syndrome (FMS) is a chronic, generalized and diffuse pain disorder accompanied by other symptoms such as emotional and cognitive deficits. The FMS patients show a high prevalence of gastrointestinal symptoms. Recently it has been found that microbes in the gut may regulate brain processes through the gut-microbiota-brain axis, modulating thus affection, motivation and higher cognitive functions. Therefore, the use of probiotics might be a new treatment that could improve the physical, psychological and cognitive state in FMS; however, no evidence about this issue is available.
Methods: This paper describes the design and protocol of a double-blind, placebo-controlled and randomized pilot study. We use validated questionnaires, cognitive task through E-Prime and biological measures like urine cortisol and stool fecal samples. The trial aim is to explore the effects of eight weeks of probiotics therapy in physical (pain, impact of the FMS and quality of life), emotional (depression, and anxiety) and cognitive symptoms (attention, memory, and impulsivity) in FMS patients as compared to placebo.
Conclusion: This pilot study is the first, to our knowledge, to evaluate the effects of probiotics in FMS. The primary hypothesis was that FMS patients will show a better performance on cognitive tasks, and an improvement in emotional and physical symptoms. These results will contribute to a better understanding in the gut-brain axis. Here we present the design and protocol of the study.
Key words: Fibromyalgia. Probiotics. Chronic pain. Clinical trial. Nutrition.
RESUMEN
Antecedentes: el síndrome de fibromialgia (FMS) es un trastorno crónico, generalizado y difuso que produce dolor acompañado de otros síntomas emocionales y cognitivos. Así mismo, los pacientes con FMS muestran una alta comorbilidad de síntomas gastrointestinales. En este sentido, recientemente se ha encontrado que la microbiota intestinal es capaz de regular procesos cerebrales a través del eje intestino-microbiota-cerebro, modulando así a nivel afectivo, emocional, motivacional y de funciones cognitivas complejas. Por lo tanto, el uso de probióticos podría ser una nueva estrategia terapéutica para mejorar el estado físico, psicológico y cognitivo en pacientes con FMS. Sin embargo, aún no hay evidencia disponible sobre este tema.
Métodos: este artículo describe el diseño y el protocolo de un estudio piloto doble ciego, controlado con placebo y aleatorizado. Se utilizan cuestionarios validados, tareas cognitivas a través de E-Prime y medidas biológicas como cortisol de orina y muestras de heces. El objetivo del estudio es explorar el efecto de un tratamiento multiespecies probióticas durante ocho semanas en la sintomatología física (dolor, impacto del FMS y calidad de vida), emocional (depresión y ansiedad) y cognitiva (atención, memoria e impulsividad) en pacientes con FMS.
Conclusión: el protocolo de este estudio piloto, a nuestro conocer, es el primero que pretende evaluar los efectos de los probióticos en FMS. La hipótesis de partida es que los pacientes con FMS mostrarán un mejor desempeño en las tareas cognitivas y una mejoría en los síntomas emocionales y físicos. Estos resultados contribuirán a una mejor comprensión del eje intestino-cerebral.
Palabras clave: Fibromialgia. Probióticos. Dolor crónico. Ensayo clínico. Nutrición.
Introduction
Fibromyalgia syndrome (FMS) is a chronic, generalized and diffuse pain disorder accompanied by symptoms such as morning stiffness or rested, fatigue, depression and sleeping disorders with unknown etiology (1,2). The global prevalence is estimated in 2.7%, with a mean prevalence of 2.5% in Europe, 3.1% in America and 1.7% in Asia (3). However, this prevalence could increase until 5.4%, with a ratio of females to males of 2.3:1 (4), using the modified 2010 criteria (5).
The FMS patients show a high prevalence of gastrointestinal symptoms (6), 81% of them reported normal alternating with irregular bowel pattern, and 63% had alternating diarrhea and constipation (7). In the same line, 32% to 80% of patients with FMS met criteria for irritable bowel syndrome (IBS), a common functional disorder of the gastrointestinal tract (8-11) with a considerable comorbidity (odds ratio 1.8-5.3) (12).
Self-reported cognitive deficits include forgetfulness, concentration difficulties, loss of vocabulary and mental slowness (13-18). Emotional and mood problems are also common in FMS. The cognitive domains that appear to be frequently affected in this population are attention, episodic memory and working memory (13,19). Interestingly, it was shown that the severity of these neurological and cognitive deficits could be related to reduced levels of Bifidobacterium and increased levels of Enterococcus spp (20). In addition, Pimentel et al. (21) found an abnormal lactulose breath test in FMS patients (78%), suggesting bacterial overgrowth of the small intestine. A posterior research of this group (22) has showed that 100% of FMS patients were diagnosed with small intestinal bacterial overgrowth compared to 84% of subjects with IBS and 20% of the healthy subjects.
All of the above show the relevance of microbiota in FMS, pointing to the potential use of probiotics in this syndrome, as has been suggested by several authors (23-25). Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (26). Probiotics has demonstrated useful in IBS (27,28) and in another patients with small intestinal bacterial overgrowth (29). In the last years, the interest in probiotics research has been increased about the effectiveness in the mood and emotional modulation (30-33) through the gut-brain axis (34-35), improving symptoms like depression, anxiety, cognitive functions, and others, and not only pain symptomatology. In fact, a recent meta-analysis shows that probiotic consumption may have a positive effect on psychological symptoms of depression, anxiety, and perceived stress in healthy human volunteers (46).
A better understanding of the probiotic effects in this pathology is required to develop integrative approaches for patient care, given the high comorbidities between FMS and gastrointestinal pathologies, as well as the high gastrointestinal symptomatology and the beneficial effect of probiotics in symptoms present in FMS. To the best of our knowledge, no randomized controlled trial has been conducted to evaluate the role of probiotics in FMS. Consequently, the aim of this research is to evaluate the effect of probiotic treatment in patients diagnosed with FMS. Therefore, in the present study, we designed a randomized controlled intervention with a multispecies probiotic to improve cognitive functions and emotional processes, as well as self-reported symptoms of depression and anxiety in FMS patients.
Material and methods
STUDY DESIGN
This is a randomized, double-blind and placebo controlled pilot study. Study enrollment took place from December 2015 to February 2016. It is designed to test whether a multispecies probiotic may improve cognition, emotional symptoms and functional state in a population diagnosed with FMS. Therefore, our aim is to explore the effects of probiotics in FMS patients as compared to placebo. Specifically, we explore the effects of probiotics in physical (e.g., pain), emotional (e.g., anxiety and depression) and cognitive (e.g., attention, memory) symptoms.
PARTICIPANTS
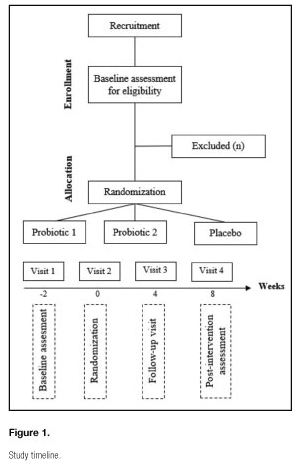
The population of our study are patients aged 18 years or older diagnosed with FMS by a physician according to the American College of Rheumatology criteria (2). This study is carried out at the University of Almeria. Participants are referred to our study from the Almeria Fibromyalgia Association (AFIAL-Spain) or the El Ejido Fibromyalgia Association (AFIEL-Spain). The inclusion criteria are: a) having been diagnosed at least one year before entering the study; b) without antibiotic treatment; and c) having signed the informed consent to participate. Exclusion criteria include: a) severe physical disability; b) being pregnant or breastfeeding; c) medication usage other than as-needed analgesics (excluding long-term narcotics); and d) meeting the criteria for psychiatric disorders other than depression and/or anxiety. The recruitment is based on an initial interview to evaluate the inclusion and exclusion criteria; this first visit is carried out two weeks before the study began. Patients do not receive any economic compensation. Figure 1 illustrates the participants' flow throughout the study.
ETHICAL ASPECTS
Informed written consent, including information about the different types of intervention (probiotics vs placebo), is obtained from all participants. This study is done in accordance with the Declaration of Helsinki, and it received ethics approval by the local Human Research Committee at the University of Almeria (Spain). The study was registered with ClinicalTrial.gov (NCT02642289).
RANDOMIZATION AND BLINDING
This study was double blinded, using identical matching placebo and probiotics. The bottles containing probiotics and placebo were identical and each one contained 60 pills; probiotics and placebo pills were indistinguishable in color, taste and smell. The group allocator, the participants, and the outcome assessor were aware of the assigned intervention.
Participants were randomly assigned to either the placebo or the interventions (probiotic) groups through a random-number generator by assignment in a 1:1:1 ratio for the three groups.
INTERVENTIONS
The intervention consisted in the administration of a multispecies probiotics. The subjects took the oral probiotics or placebo every day for eight weeks. The doses were four pills each day. The three different interventions are identical in appearance, taste and smell.
-Probiotic 1: the oral probiotic 1, consisted of S. thermophiles, S. faecium, L. acidophilus, L. rhamnosus, L. casei, L. bulgaricus, B. bifidum, and B. infantis. This probiotic was provided by the manufacturer Complementos Fitonutricionales (CFN. S.L., Granada, Spain) through their product Probiotic 5000. Each capsule contains about five million bacteria.
-Probiotic 2: the oral probiotic 2 was Ergyphilus Plus, provided by the manufacturer Nutergia (Spain), containing six million revivifications of germs per capsule. This probiotic contains the following bacterial strains: L. Rhamnosus GG®, Casei, Acidophilus, and B. Bifidus.
-Placebo: the placebo was composed by cellulose, an inert substance, and was provided by the manufacturer Complementos Fitonutricionales (CFN. S.L., Granada, Spain). The duration and the daily pills intake was the same than in the probiotics groups.
INTERVENTION TIMELINE
The study timeline is shown in figure 1 and intervention details are outlined below.
-Study visit 1: a recruitment visit was carried out two weeks before the beginning of the study to evaluate the inclusion an exclusion criteria.
-Study visit 2: at the second visit, week zero, the subjects were enrolled following informed consent, and randomized to probiotic or placebo. Initial stool and urine samples were collected. Also, all the demographic information was taken. This visit is considered as the baseline pre-treatment and all the outcomes were measured.
-Study visit 3: after four weeks of treatment we carried out a visit (visit 3) to evaluate adherence.
-Study visit 4: eight weeks after, in the fourth visit, the subject stopped taking the probiotic or placebo. This visit is considered as the post-treatment effects and all the outcomes were measured. Stool and urine samples were collected.
ADHERENCE
Subjects were followed through weekly by telephone calls to identify side effects from the medication and any medication-related problems. In addition, after four weeks of treatment an interview with the patients was carried out to evaluate adherence to the treatment. Tolerability is an important factor in subject adherence to get a potentially beneficial intervention.
SUBJECT WITHDRAWAL
All subjects were informed during enrollment that they may discontinue participation at any time.
OUTCOME MEASURES
The primary outcome of this study is FMS patients cognition improves after eight weeks of treatment. We hypothesize that FMS patients will show a better performance on impulsive choice and decision-making, as well as on working memory and attentional control, following the treatment with probiotics. As secondary outcomes, we hypothesize mean changes between baseline and after intervention on body composition, pain, and emotional outcomes. Additional secondary outcomes include changes on physiological measures (free cortisol concentration in urine and stool fecal samples).
ASSESSMENT TOOLS
The following assessment tools were used:
1. Demographic information: gender, age, time with fibromyalgia, and years of formal education.
2. Anthropometric measurements of body mass index and corporal composition (fat mass and lean mass) were assessed by bioelectric impedance analyzer of six electrodes (Electro Interstitial Scanner, LD Technology, Miami, FL).
3. A Spanish validated version of several questionnaires was used to evaluate several symptoms or dimensions of FMS patients.
-Pain: Visual Analogue Scale (47) and McGuill Pain Questionnaire (48).
-Quality of life: Short-Form Health Survey (SF-36) (49).
-Impact and severity of FMS symptoms: Fibromyalgia Impact Questionnaire (50).
-Sleep quality: Pittsburg Sleep Quality Index (51).
-Emotional symptoms: depression (Beck Depression Inventory [52]) and anxiety (State-Trait Anxiety Inventory [53]).
-Cognitive dimension: general evaluation (Mini-Mental State Examination [54]) and verbal working memory (Digits [55]).
4. The following computerized cognitive tasks were used. E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) controlled the presentation of the stimuli as well as collection of the participant's responses in the tasks:
-Working memory: Directed-forgetting Task, Corsi Task, Simon Task, and N-Back Task.
-Inhibitory control mechanisms of attention: Go/No-Go Task, Distractor Devaluation Effect Task, and Stroop Task with negative priming.
-Impulsive choice and decision-making: Iowa Gambling Task, Task Switching and Two-Choice Task.
5. Free cortisol in urine, using a competitive ELISA kit (RE5224, Cortisol ELISA, IBL International, Hamburg, Germany).
6. Stool fecal samples, using QIAampR DNA Stool Mini Kit (QIAGEN) to extract DNA, and PCR-R to analyze the DNA.
ADVERSE EVENTS
-Safety of probiotics: Lactobacillus and Bifidobacterium species have long been considered as safe for human consumption, and their safety has been studied in many publications (56). They were made commercially available many years ago. In our study, our products have been supplied by CFN. S.L. and Nutergia, which commercialize them reliably.
-Safety of placebo: the placebo agent was composed of cellulose, an inert, inactive substance. Being an inert substance, the likelihood for adverse reaction is virtually none, and there is no obvious foreseeable risk associated with the ingestion of the placebo capsules.
DATA MANAGEMENT
Research data is handled with utmost confidentiality and discretion. Subjects are assigned a unique identification number that can be traced only by the research specialist and PI. The file linking study identification numbers to identifiable information is stored and secured separately from the coded data. All subject information is kept in locked drawers, file cabinets or secure computer files, with access only allowed to research personnel. The final trial dataset will be available to all research personnel.
STATISTICAL ANALYSIS
-Sample size justification. We conducted a randomized controlled trial with probiotic and placebo randomized in a 1:1:1 manner. Because this is a pilot feasibility study, a sample size calculation was not conducted as there are not previous studies evaluating probiotics in FMS. We considered that it was feasible to recruit an overall of 60 participants, with 20 patients randomized to each group.
-Data analysis. For baseline demographic data, questionnaires and behavioral measurement, the mean scores (total and/or partial) were calculated using descriptive statics. Repeated measures analysis of variance (ANOVA) was used to evaluate the significance of the participants' evolution within each group by comparing the different scores at various time points compared to baseline. Even though the data show a non-normal distribution, Friedman test were used. A p-value of < 0.05 is considered as statistically significant. In addition to the statistical significance levels, the effect size estimates were calculated by η2 (eta-squared). This indicator provided estimates of the magnitude of the effects that are independent of the sample size. Statistical analysis was performed using software SPSS for Windows, Version 21.0 (SPSS Inc., Chicago, Illinois, USA).
Discussion
Ingestion of probiotics positively affects the host's health by improving intestinal the microbial balance (57), and also may positively affect cognitive and psychological processes via the gut-brain-axis (44). The proposed mechanisms of action of probiotics include competition against the pathogenic bacteria to bind to the intestinal epithelial cells, enhancement of the intestinal epithelial barrier function, inhibition of pathogens' growth by secretion of antimicrobial peptides, and augmentation of the production of serum IgA (58,59). Probiotics may also affect the central nervous system via the gut-brain axis by: a) enhancing the production and delivery of neuroactive substances, such as gamma-amino butyric acid (GABA), serotonin, dopamine and acetylcholine; b) the vagus nerve (60); and c) decreasing pro-inflammatory cytokines, which are able to cross the blood-brain barrier and elicit mood and behavioral changes (58). For instance, some probiotics have been shown to induce an elevation of tryptophan levels in plasma (61), which is a precursor of serotonin. Serotonin has been implicated in emotional processes, cognition, motor function and pain, as well as in neuroendocrine functions, such as food intake, circadian rhythms and reproductive activity (62). A very important practical implication of this is that probiotics may then produce health benefits in patients suffering from neuropsychiatric disorders (60). Therefore, FMS is one of the target population. These patients show a seemingly altered GI microbial community (20,63). Their symptoms include chronic, generalized and diffuse pain disorders, as well as fatigue, depression, sleeping disorders (1), self-reported reduced mental performance (14-18) and cognitive deficits in inhibition and decision-making processes (64).
Previous studies with probiotics in population with a great comorbidity with FMS, like CFS and IBS (6,11,12,65), have reported beneficial effects after treatment in quality of life (60), pain severity (67), reduced inflammation (68), and emotional symptoms like anxiety (69).
To our knowledge, the current pilot study is the first randomized controlled trial evaluating the effects of probiotics on FMS. This study will identify the potential effect of probiotics in several dimensions of FMS, since these effects are still unknown. Moreover, this protocol study will provide a complex and a large evaluation of several symptoms in FMS, affecting to the physical, emotional and cognitive symptoms. Thus, it could affect to the quality of life of patients with FMS, improving some symptoms that limited their daily life.
While this study adds important knowledge to the literature, it does have some limitations. The primary limitation of this study is the sample size. This was designed as a pilot study, planned to assess feasibility, attrition, completion of study procedures, and recruitment challenges, prior to undertaking a larger study. Second, dietary measures were not included and consumption of other fermented foods (e.g., yogurt) was not controlled. Hence, we cannot exclude that the consumption of probiotics will be accompanied by spontaneous dietary changes that may have indirectly accounted for the observed effects.
Despite the limitations, the results of this study will provide the first evidence of the effect of probiotics on FMS. In addition, data will have important clinical implications. Given that neither pharmacological nor non-pharmacological interventions are effective on FMS by themselves, a multidisciplinary intervention including both of them is the most effective approach so far (70).
Acknowledgements
PR is supported by a pre-doctoral grant by the Research Plan by the University of Almeria. In addition, we wish to thank the staff of the Almeria Fibromyalgia Association (AFIAL-Spain) and the El Ejido Fibromyalgia Association (AFIEL-Spain) for their help throughout the development of this study.
ClinicalTrial.gov Identifier: NCT02642289
References
1. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33(2):160-72. [ Links ]
2. Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600-10. [ Links ]
3. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 2013;17(8):356. [ Links ]
4. Jones G, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol 2015;67(2):568-75. [ Links ]
5. Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38(6):1113-22. [ Links ]
6. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160(2):221-7. [ Links ]
7. Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci 1991;36(1):59-64. [ Links ]
8. Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser A-S, Klapp BF, et al. Somatic comorbidities of irritable bowel syndrome: A systematic analysis. J Psychosom Res 2008;64(6):573-82. [ Links ]
9. Sperber AD, Atzmon Y, Neumann L, Weisberg I, Shalit Y, Abu-Shakrah M, et al. Fibromyalgia in the irritable bowel syndrome: Studies of prevalence and clinical implications. Am J Gastroenterol 1999;94(12):3541-6. [ Links ]
10. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterol 2002;122(4):1140-56. [ Links ]
11. Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001;134(9 Pt 2):868-81. [ Links ]
12. Kanaan RAA, Lepine JP, Wessely SC. The association or otherwise of the functional somatic syndromes. Psychosom Med 2007;69(9):855-9. [ Links ]
13. Glass JM, Park DC. Cognitive dysfunction in fibromyalgia. Curr Rheumatol Rep 2001;3(2):123-7. [ Links ]
14. Glass JM, Park DC, Minear M, Crofford LJ. Memory beliefs and function in fibromyalgia patients. J Psychosom Res 2005;58(3):263-9. [ Links ]
15. Glass JM. Fibromyalgia and cognition. J Clin Psychiatry 2008;69(Suppl 2):20-4. [ Links ]
16. Glass JM. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: New trends and future directions. Curr Rheumatol Rep 2006;8(6):425-9. [ Links ]
17. Glass JM. Review of cognitive dysfunction in fibromyalgia: A convergence on working memory and attentional control impairments. Rheum Dis Clin North Am 2009;35(2):299-311. [ Links ]
18. Glass JM, Williams DA, Fernández-Sánchez ML, Kairys A, Barjola P, Heitzeg MM, et al. Executive function in chronic pain patients and healthy controls: Different cortical activation during response inhibition in fibromyalgia. J Pain 2011;12(12):1219-29. [ Links ]
19. Gelonch O, Garolera M, Rosselló L, Pifarré J. Cognitive dysfunction in fibromyalgia. Rev Neurol 2013;56(11):573-88. [ Links ]
20. Butt H, Dunstan R, McGregor N, Roberts T. Bacterial colonosis in patients with persistent fatigue. In: Proceedings of the AHMF international clinical and scientific conference; 2001. [ Links ]
21. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2003;98(2):412-9. [ Links ]
22. Pimentel M, Wallace D, Hallegua D, Chow E, Kong Y, Park S, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis 2004;63(4):450-2. [ Links ]
23. Galland L. The gut microbiome and the brain. J Med Food 2014;17(12): 1261-72. [ Links ]
24. Slim M, Calandre EP, Rico-Villademoros F. An insight into the gastrointestinal component of fibromyalgia: Clinical manifestations and potential underlying mechanisms. Rheumatol Int 2015;35(3):433-44. [ Links ]
25. Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond) 2010;7(1):79. [ Links ]
26. FAO/WHO. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Prebiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Vol. 85, Food and Nutrition Paper; 2001. p. 71. [ Links ]
27. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol 2015;21(10):3072-84. [ Links ]
28. Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of Lactobacillus in treating irritable bowel syndrome: A meta-analysis. Intern Med 2015;54(3): 243-9. [ Links ]
29. Khalighi AR, Khalighi MR, Behdani R, Jamali J, Khosravi A, Kouhestani S, et al. Evaluating the efficacy of probiotic on treatment in patients with small intestinal bacterial overgrowth (SIBO) - A pilot study. Indian J Med Res 2014;140(5):604-8. [ Links ]
30. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 2007;61(3):355-61. [ Links ]
31. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105(5):755-64. [ Links ]
32. Steenbergen L, Sellaro R, Van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015;48:258-64. [ Links ]
33. Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011;2(4):256-61. [ Links ]
34. Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 2016;32(2):96-102. [ Links ]
35. De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J Physiol 2014;592(14):2989-97. [ Links ]
36. Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 2008;29(1): 117-24. [ Links ]
37. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol 2015;28(2):203-9. [ Links ]
38. Huang R, Wang K, Hu J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016;8(8). [ Links ]
39. Bravo JA, Forsythe P, Chew M V, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011;108(38):16050-5. [ Links ]
40. Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 2014;817:221-39. [ Links ]
41. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes 2014;5(3):404-10. [ Links ]
42. Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motil 2011;23(3):187-92. [ Links ]
43. Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13(10):701-12. [ Links ]
44. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 2011;108(7):3047-52. [ Links ]
45. Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381-96. [ Links ]
46. McKean J, Naug H, Nikbakht E, Amiet B, Colson N. Probiotics and subclinical psychological symptoms in healthy participants: A systematic review and meta-analysis. J Altern Complement Med 2016;acm.2016.0023. [ Links ]
47. Huskisson EC. Measurement of pain. Lancet 1974;2(7889):1127-31. [ Links ]
48. Lahuerta J, Smith B, Martínez-Lage A. An adaptation of the McGill Pain Questionnaire to the Spanish language. Schmerz 1982;(3):132-4. [ Links ]
49. Alonso J, Prieto L, Antó JM. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results. Med Clin 1995;104(20):771-6. [ Links ]
50. Monterde S, Salvat I, Montull S, Fernández-Ballart J. Validación de la versión española del Fibromyalgia Impact Questionnaire. Rev Esp Reumatol 2004;31(9):507-13. [ Links ]
51. Macias J, Royuela A. La versión española del índice de calidad del sueño de Pittsburg. Inf Psiquiatr 1996;(146):465-72. [ Links ]
52. Conde V, Useros E. Adaptación castellana de la escala de evaluación conductual para la depresión de Beck. Rev Psiquiat Psicol Med Eur Am Lat 1975;(12):217-36. [ Links ]
53. Seisdedos N. Cuestionario de Ansiedad Estado-Rasgo (STAI). Madrid: TEA; 1982. [ Links ]
54. Lobo A, Escobar V, Ezquerra J, Seva Díaz A. El Mini-Examen Cognoscitivo. Un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes psiquiátricos. Rev Psiquiatr Psicol Med 1980;14(5):39-57. [ Links ]
55. Ibor J. Escala de inteligencia de Wechsler para adultos III. Schizophr Res 2005;(78):147-56. [ Links ]
56. Mäkelä R, Mäkilä H, Peltomaa R. Dietary therapy in patients with inflammatory arthritis. Altern Ther Health Med 2017;23(1):34-9. [ Links ]
57. Dinan TG, Quigley EM. Probiotics in the treatment of depression: Science or science fiction? Aust N Z J Psychiatry 2011;45(12):1023-5. [ Links ]
58. Hardy H, Harris J, Lyon E, Beal J, Foey AD. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013;5(6):1869-912. [ Links ]
59. Upadhyay N, Moudgal V. Probiotics: A review. J Clin Outcomes Manag 2012; 19(2):76-84. [ Links ]
60. Dinan TG, Stanton C, Cryan JF. Psychobiotics: A novel class of psychotropic. Biol Psychiatry 2013;74(10):720-6. [ Links ]
61. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008;43(2):164-74. [ Links ]
62. Martinowich K, Lu B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacol 2008;33(1):73-83. [ Links ]
63. Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol 2008;24(1):11-6. [ Links ]
64. Verdejo-García A, López-Torrecillas F, Calandre EP, Delgado-Rodríguez A, Bechara A. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol 2009;24(1):113-22. [ Links ]
65. Buchwald D, Garrity D. Comparison of patients with chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivities. Arch Intern Med 1994; 154(18):2049-53. [ Links ]
66. Lorenzo-Zúñiga V, Llop E, Suárez C, Álvarez B, Abreu L, Espadaler J, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol 2014;20(26):8709-16. [ Links ]
67. Francavilla R, Miniello V, Magista AM, De Canio A, Bucci N, Gagliardi F, et al. A randomized controlled trial of Lactobacillus gg in children with functional abdominal pain. Pediatrics 2010;126(6):e1445-52. [ Links ]
68. Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012;61(3):354-66. [ Links ]
69. Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog 2009;1(1):6. [ Links ]
70. Nüesch E, Häuser W, Bernardy K, Barth J, Jüni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: Network meta-analysis. Ann Rheum Dis 2013;72(6):955-62. [ Links ]
![]() Correspondence:
Correspondence:
Diana Cardona Mena.
Department of Nursing Science, Physiotherapy and Medicine.
Edificio de Ciencias de la Salud.
Universidad de Almería.
Ctra. Sacramento, s/n.
04120 La Cañada de San Urbano.
Almería, Spain.
e-mail: dcardona@ual.es
Received: 23/05/2017
Accepted: 06/06/2017