INTRODUCTION
Oral cancer (OC) is the sixth most prevalent human cancer in the world and in Latin America (1,2). Globally, the vast majority of these are oral squamous cell carcinomas (OSCC) (1). OC is associated with high morbidity and low survival rates. Most cancers, including OC, have complex pathologies, and their incidence and survival are closely related to the social, cultural and socio-economic determinants of health (3,4). Some of these factors, such as diet, are modifiable (5).
Controversies abound in epidemiological studies on the relationship of diet and oral carcinogenesis (5,6). Many studies indicate that red meat intake or a diet poor in fruit and vegetables could increase the risk of different cancers such as OSCC (6), probably because certain dietary components are related to inflammatory processes (7). Chronic local inflammation is known to disturb homeostatic control in cell signaling pathways, which may transform normal cells to become premalignant or malignant. In some kinds of cancer, an inflammatory environment is present before malignant change occurs (8). However, in other kinds of cancer, malignant change induces an inflammatory environment around a primary lesion and promotes tumor development (9). Inflammatory processes are known to play a major role at different stages of tumorigenesis (9), and chronic inflammation predisposes to develop cancer (10). There is evidence that various dietary components could lead to chronic inflammation (11). For this reason, it is interesting to evaluate the association between dietary inflammatory potential and OSCC.
Researchers at the University of South Carolina’s Cancer Prevention and Control Program have developed a tool called the Dietary Inflammatory Index (DII) to measure the overall inflammatory potential of any individual’s diet (12). The DII has been validated in numerous studies and has been shown to be associated with different kinds of cancers and other diseases (12-15). However, there are few studies on the association between DII and OC (16), and this relation has not yet been studied in Argentinian people with OSCC. Hence, the aim of this study was to evaluate if there is an association between dietary inflammatory potential and OSCC in adult patients. The results of this study could have direct applicability in programs to prevent OSCC.
MATERIAL AND METHODS
A case-control study with controls in a 3:1 ratio to cases and representing both genders was carried out between 2012 and 2015. The clinical examination of the oral cavity was performed by previously trained dentists. Lifestyle habits were assessed using the criteria by Secchi et al. 2015 (6). The medical, dental characteristics and dietary intake data were collected in a unique clinical record.
All cases (n = 27) were ≤ 85 years old at diagnosis (age range, 23-83 years; mean, 59 years) and were recruited at the outpatient clinic, Odontology School, Universidad Nacional de Córdoba, Argentina. A total of 27 patients, newly diagnosed by histopathological analysis and classified by the International Classification of Diseases (ICD-10) codes C00 to C14, were considered eligible for the study. Controls (n = 86) were enrolled in the same period and at the same clinic as cases. They presented at the time of the survey with a mean age of 59 years and an age range between 21 and 86 years. They did not have any neoplastic diseases, and they did not report changes in their dietary habits or other relevant habits such as smoking and drinking for a period of no less than 5 years. They were matched by gender and age (± 5-years) with cases.
Additional risk factors were assessed according to the following criteria: smoker: current consumption of at least one cigarette⁄day over a 1-year minimal period; alcohol: current consumption of 2 drinks⁄week over a 1-year minimal period. The workplace (e.g., occupational exposure to carcinogens) presents possible risks in industries such as textiles, rubber, coal, dyes, leather, herbicides, automotive, plastics and chemicals. Age was categorized as < 45 years and ≥ 45 years. The E-DII score was considered a continuous variable or categorized into tertiles based on cutpoints among controls. Age was fit as a continuous variable. Body mass index, calculated as BMI = weight (kg)/height (m) was categorized into < 25 or ≥ 25 kg/m2 according to World Health Organization criteria (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Education was classified as primary, secondary, tertiary, and university.
DIETARY ASSESSMENT
A 127-item food frequency questionnaire (FFQ), validated by Navarro et al., 2001 (17), was administered to cases and controls by trained nutritionists at the stomatology clinic after clinical examination and histopathological confirmation of clinical diagnosis.
This questionnaire includes two sections: a) bio-socio-cultural characteristics, anthropometric measurements, and lifestyle; b) food intake, evaluating dietary exposure in the 5 years prior to diagnosis for cases, and before interview for controls. Additionally, a photographic food atlas, also validated by Navarro et al., 2000 (18), was used, and nutritional composition was estimated using the Nutrio 1.2 sofware package (19).
DIETARY INFLAMMATORY INDEX ASSESSMENT
Details of the steps involved in the DII calculation are described elsewhere (20,21). In order to compute the DII score, the dietary information for each study participant was first linked to the regionally representative database, which provided a robust estimate of a mean and standard deviation for each of the 45 parameters (i.e., foods, nutrients, and other food components) considered in the DII definition (20,21). These parameters were then used to derive the subject’s exposure relative to a standard global mean as a z-score, by subtracting the mean of the regionally representative database from the amount reported, and dividing this value by the parameter’s standard deviation. To minimize the effect of “right skewing”, this value was converted to a centred percentile score, which was computed by doubling the raw percentile score and then subtracting 1. This score was then multiplied by the respective food parameter effect score (derived from a literature review of 1943 articles) (21). All of these food parameter-specific DII scores were then summed to create the overall DII score for every subject in the study. The energy adjusted-DII (E-DII) was calculated per 1000 kcal using methods paralleling those of the DII, but relied on an energy-adjusted global database. A higher E-DII score indicates a pro-inflammatory diet rich in calorie-dense nutrients such as saturated fat and total cholesterol; a lower E-DII score indicates that the diet is more anti-inflammatory, rich in nutrients such as vitamins, minerals, and a variety of other antioxidant compounds (16,22).
ETHICAL ASPECTS
This study was approved by the Research and Ethics Committee of the Ministry of Health of the Province of Córdoba (No. 1378), and all subjects signed informed consent forms. Patients who were under therapeutic medication such as corticosteroids or chemotherapy drugs that modify or alter the clinical behavior of malignant oral lesions were excluded. Patients diagnosed with other cancers, systemic diseases, chronic alcoholism or drug addictions were also excluded.
STATISTICAL ANALYSIS
Quantitative data were statistically described using mean ± standard error and median values. Qualitative variables were described as relative/absolute frequencies. The Mann-Whitney test for testing the hypothesis that median consumption is equal between cases and controls was performed because continuous data were not normally distributed. The measures of association, the odds ratio (OR) and its 95% confidence interval (95% CI), were estimated by fitting the logistic regression model between the presence of disease and the E-DII score, while controlling for potential confounders. For all tests, statistical significance was set at p < 0.05 (2-sided). The Stata Statistical Software package, version 13 (Stata Corp LP -2014- College Station, TX 77845, USA), was used for all analyses.
RESULTS
Patients with OSCC presented with lesions in various oral cavity sites: tongue (42.1%), palate (6.3%), lip (9.6%), oral mucosa (16.2%), gum (16.2%), and floor of the mouth (9.6%). Most of the patients were diagnosed within one year of the first symptoms appearing (80%). Cases and controls showed similar distributions of bio-demographic characteristics such as BMI. They were matched on age and gender (Table I).
Table I. Bio-demographic and risk factor characteristics studied. Absolute and relative % frequencies (RF calculated over total of controls or cases)

1Body mass index (BMI): weight (kg)/height (m)2.
2Smoker: current consumption of at least one cigarette⁄day over a 1-year minimal period.
3Alcohol: current consumption of 2 drinks⁄week over a 1-year minimal period.
4Work: one or more years of exposure to carcinogens considered a risk factor at work in industries such as textiles, rubber, leather, herbicides, automotive, plastic, and chemicals by IARC. p-values < 0.05 indicate statistical significance.
Cases had a significantly higher intake of fat and protein as compared to controls (Table II). Other dietary components such as cholesterol, iron, monosaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), omega-6 fatty acids, omega-3 fatty acids, selenium, vitamin B6, and vitamin E were significantly higher in cases than in the control group (Table II). The ratio of omega-6/omega-3 fatty acids in controls (average, 8.49 ± 3.9) was significantly lower than in cases (average, 10.44 ± 4.9) (p = 0.025, Mann Whitney test) (Table II).
Table II. Macronutrients and micronutrients including estimation of energy-adjusted dietary inflammatory index (E-DII) scores in cases and controls

MUFA:monosaturated fatty acid;
PUFA:polyunsaturated fatty acid.
aMann-Whitney U-test for proving Ho: median values are equal between cases and controls. Bold letters indicate statistical significance at p < 0.05.
The median E-DII score was 0.81, with a range of -2.24 to +3.72 across the entire population. A significant association was observed between E-DII (continuous) and OSCC by logistic regression (OR 1.69, 95% CI [1.18-2.43]) after adjusting for alcohol and tobacco consumption, the two main parameters recognized as risk factors for oral cancer (Table III). A similar result was observed with E-DII categories based on tertiles (Table III).
Table III. The relationship between E-DII scores and oral squamous cell carcinoma from a logistic regression model
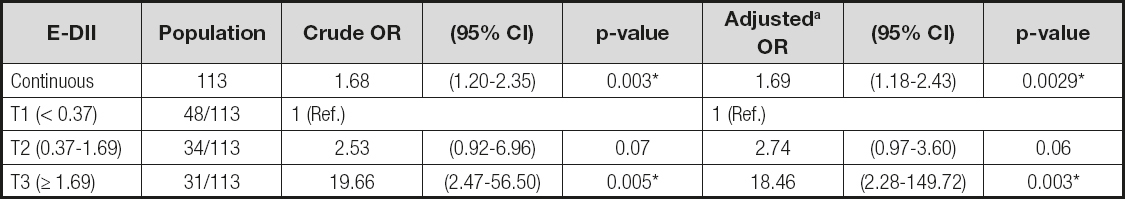
OR: odds ratio; CI: confidence interval.
aT1: DII values lower than the 1st tertile; T2: E-DII values between 2nd and 3rd tertile; T3: E-DII values higher than 3rd tertile. Categories based on E-DII tertiles of controls. Adjusted by alcohol and tobacco consumption.
*Indicates statistical significance at p < 0.05.
DISCUSSION
The results of this study showed that OSCC cases had significantly higher daily intakes of fat and protein than control subjects. It has previously been shown that factors such as growth hormones and estrogen can modify metabolic factors, adipocytokine, low-grade inflammation, and cellular oxidative stress levels, and can also result in alterations of the microbiome, which may eventually lead to carcinogenesis (23). It has been demonstrated that NFκB signaling and expression of COX-2, TNFα and IL-1β are all increased in the mammary glands and visceral fat of obese female mice obtained through genetic and diet-induced obesity models; these molecules participate in processes associated with chronic inflammation and have been linked to various epithelial malignancies (24).
A significantly higher intake of iron was observed in cases with OSCC vs. controls in this study. This observation matches other studies, demonstrating a relationship between this micronutrient and the presence of multiple cancer types, such as lung cancer, breast cancer, prostate cancer, colorectal cancer, hepatocellular cancer, pancreatic cancer, hematological cancer, renal cell carcinoma, and melanoma (6,25). One explanation is that iron has important functions in mammalian cells, such as cell proliferation, metabolism and growth (6,25). Iron- and heme-containing proteins, including the enzymes involved in DNA stability and cell cycle progression, the mitochondrial enzymes implicated in respiratory complexes, and detoxifying enzymes such as peroxidase and catalase, control these processes (25). For example, heme-iron can promote endogenous production of nitrous compounds and catalyze free radical formation, leading to oxidative cell damage (25). Iron is one of the pro-inflammatory components in the DII.
A high intake of omega-6 and PUFA was observed in this study in patients with OSCC compared with control subjects. Omega-6 metabolism produces arachidonic acid (AA), which makes inflammatory prostaglandins and lipoxins by oxidation. In contrast, sources of omega-3 have anti-inflammatory activities (26). There is evidence that food components such as α-linolenic acid, omega-3 and omega-6 PUFAs, conjugated linoleic acid, butyrate, curcumin, resveratrol, genistein, vitamin A, vitamin D, etc., can regulate inflammatory processes (26,27).
Our results showed a higher ratio of omega-6 to omega-3 fatty acids in oral cancer patients. Our previous experimental work showed that tumors of DMBA-induced mice fed chia oil (enriched omega-3) reduced the ω-6/ω-3 ratio and decreased tumor development (28). The relationship between omega-6 and omega-3 could modify the action of carcinogenic factors and decrease the risk of oral cancer development (28). In Western populations the ω-6/ω-3 ratio varies from 10:1 to 20-25:1, while in populations such as the Japanese this ratio is much lower, i.e., around 4:1 (29). In vivo and in vitro animal studies have shown that the balance between ω-6 and ω-3 PUFAs has an impact on the development of cancers such as prostate cancer (29). In human feeding studies with fish or fish oil, EPA and DHA partially replace ω-6 PUFAs, especially AA, probably in the membranes of all cells (27). Given that in Western diets the amount of ω-6 is greater than in other populations, eicosanoids obtained from the metabolic pathway of AA are present in greater quantities than those produced by ω-3 PUFAs (28). It is known that LA and ALA are not exchangeable compounds that compete for Δ6-desaturase, an enzyme that participates in the elongation of the hydrocarbon chains of PUFAs. In addition, it is known that the activity of desaturases Δ6 and Δ5 is the main factor controlling the conversion of dietary LA to AA (28).
In our study we also observed that selenium (Se) intake was increased in patients with OSCC. Experiments in vivo, in vitro, and in healthy persons have shown that Se is involved in the regulation of epigenetic mechanisms (30). These studies have determined that high exposure to Se leads to inhibition of DNA methyltransferase activity, and affects methylation of specific tumor suppressor genes, among other pathways studied (30).
Several of the dietary components observed in this study participate in epigenetic events. These include vitamin B6, omega-6 and omega-3 PUFAs, and Se, among others. The dietary modulation of the epigenome is related to the processes involving the metabolism of one-carbon moieties. Methylation reactions catalyzed by methyltransferases depend on a set of methyl-S-adenosylmethionine (SAM) amino acids in the human body. Methyl-tetrafolate is a methyl donor group that converts homocysteine to methionine (30). Methionine activates SAM by means of methionine-adenosyltransferase, adding a methylated cytosine group. This addition of the methyl group is a complex process that can be affected by several dietary factors including folate, methionine, and several B vitamins (B2, B6 and B12). This is why the factors consumed in the diet that are related to the methyl group can participate in epigenetic changes (31,32).
Our study showed a relationship between E-DII and risk of OSCC. Other cancers such as gastric, prostate and colorectal cancer also have evinced this relationship (33 34-35). It is known that inflammation is associated with the development of most cancers; and the inflammatory tumor microenvironment is related to several factors such as infections, tobacco smoking, and excessive alcohol consumption, all of which increase cancer risk and encourage malignant progression (1,6,8). In a study conducted in Japan a positive association was observed between increasing E-DII scores and overall upper aerodigestive tract cancers, as well as across anatomic subsites. For upper aerodigestive tract cancers the ORQ4vsQ1 was 1.73 (95% CI: 1.37-2.20); for head and neck cancer the ORQ4vsQ1 was 1.92 (95% CI: 1.42-2.59); and for esophageal cancer the ORQ4vsQ1 was 1.71 (95% CI: 1.54-1.90). The risks for hypopharyngeal and nasopharyngeal cancers were greatly elevated: ORQ4vsQ1 = 4.05 (95% CI: 1.24-13.25) for hypopharyngeal cancer and ORQ4vsQ1 = 4.99 (95% CI: 1.14-21.79) for nasopharyngeal cancer (36).
The limitations of the present study include a small number of cases. The small sample size might result in unstable risk estimates with wide confidence intervals. In addition, the range of E-DII scores was much narrower than seen in other studies (i.e., ≈6 compared to ≈11) (37). Another limitation includes the retrospective character of the case-control design, which is prone to both selection and information biases (38,39).
In conclusion, our results are inconsistent with the null hypothesis of no association between DII and OSCC, suggesting that the inflammatory components of daily diet could be involved in the development of OSCC. These results are consistent with the biological mechanisms described in both experimental and observational studies in OSCC and other types of cancers. There is a need for future research on this topic, including larger studies, especially those with prospective design, to explore the association between dietary inflammatory potential and oral premalignant and malignant lesions.














