INTRODUCTION
Stroke is a common acute cerebrovascular disease. According to its etiology, stroke can be divided into ischemic stroke, hemorrhagic stroke, and other types of stroke. The incidence rate of ischemic stroke is approximately 2-3 times higher than that of hemorrhagic stroke (1). Treatment strategies for stroke vary based on its etiology, and the main methods for treating ischemic stroke include thrombolysis, mechanical thrombectomy, anti-platelet aggregation, reducing blood lipid levels, and promoting the recovery of neurological function. In addition to drug treatment, nutrient support is also an important approach for treating hemorrhagic stroke. Stroke is associated with multiple serious complications, such as pulmonary infection and deep venous thrombosis (DVT), and is also often accompanied by motor disorders, swallowing disorders, sensory disorders, and other sequelae.
The mortality and morbidity of stroke have decreased in recent years, but stroke remains the second leading cause of death and disability worldwide (2,3). The increasing number of strokes has caused a substantial burden for patients, their families, and society. Due to the aging of the population and insufficient management of risk factors, this burden is expected to be further increased. Hence, we need to explore novel treatments for stroke (4,5).
n-3 polyunsaturated fatty acids (n-3 PUFAs) have various pharmacological activities, such as anti-inflammatory, anti-thrombosis, and anti-atherosclerosis activities (6-9). Among the many risk factors for cardiovascular and cerebrovascular diseases, abnormal blood lipid levels and lipid metabolism disorders play an important role (10). N-3 PUFAs can reduce blood lipid levels and can significantly improve endothelial dysfunction (11-13).
It is unclear what role n-3 PUFAs play in stroke and whether they have a beneficial effect on stroke recovery and prognosis. This study aims to systematically analyze the correlation between n-3 PUFAs and stroke via a meta-analysis to provide evidence-based medicine for the prevention and treatment of stroke.
METHODS
This analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (14). All the analyses involved were performed according to the methods described in previously published studies. Thus, no ethical approval or patient consent was needed.
DATA SOURCES AND SEARCH STRATEGY
We searched the PubMed, EMBASE, Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI) databases from inception to January 1, 2022. The search terms were as follows: “Fatty Acids, Omega-3” or “Omega-3 Fatty Acid” or “n-3 Fatty Acids” or “n3 Polyunsaturated Fatty Acid” or “n-3 PUFA” or “stroke” and “cerebrovascular Accident” or “cerebrovascular apoplexy” or “brain vascular accident” or “cerebral stroke” or “CVA ” or “acute cerebrovascular accident”. We used the combination of subject words and free words to perform the search and logical symbols, wildcards, and Boolean logic operators to write the search expressions. No language or geographic restrictions were applied.
STUDY SELECTION
The studies were included if they met the specific criteria for this review: 1) the study design was RCT; 2) the participants were subjects with stroke or high risk of cerebrovascular accident; and 3) the study intervention was n-3 PUFAs, and the comparator was placebo or another therapy.
Exclusion criteria were as follows: a) the quality of the study was poor, including serious flaws in research design and unclear indicators of outcome; b) the article type was a case report, case series, animal study, in vitro study, meeting minutes, or review; and c) no access to the original text, raw data, or further relevant information.
DATA EXTRACTION AND OUTCOMES
Two authors (Du LL and Gu H) independently screened the searched literature according to inclusion and exclusion criteria, extracted data, and discussed their findings with each other. Controversial studies were evaluated for inclusion by the third author (Xu Q).
One author (Xu Q) extracted the data, including surname of first author, numbers of participants, publication year, country, and outcome data from the intervention group and control group of the included trials. Our outcomes of interest included: 1) vascular disease-related death; 2) cerebrovascular events; 3) biochemical indices, such as lipid profiles (total cholesterol [TC], triglycerides [TG], high-density lipoprotein cholesterol [HDL], and low-density lipoprotein cholesterol [LDL]); 4) psychological cognition scales, such as the SF-36 questionnaire, General Health Questionnaire (28 items), CVLT (California Verbal Learning Test); and neurological assessment, such as BI, RMI, Nagi Scale, Rosow-Breslau Scale, and Katz ADL scale. If the articles did not provide adequate data for analysis, we contacted the authors to request detailed information. Another author (Ji M) reviewed these data.
RISK OF BIAS ASSESSMENT
Two authors (Du LL and Gu H) used Cochrane's tool (15) to evaluate the risk of bias of the included trials, and a third author (Xu Q) was responsible for confirming their judgments. Seven domains were judged as having high, unclear, or low risk of bias in the RCTs: allocation concealment, blinding of participants and personnel, random sequence generation, selective reporting, blinding of outcome assessors, incomplete outcome data, and other biases. If a study did not provide data about AEs, it was considered to have an unclear risk of selective reporting bias.
STATISTICAL ANALYSIS
This meta-analysis was performed with Stata 15.1 (Stata Corporation). Data were used as input and double-checked by two reviewers. We anticipated clinical heterogeneity and thus chose the random-effects model. Dichotomous variables are presented as risk ratios (RRs) with 95 % confidence intervals (CIs). Continuous variables are presented as the mean difference or standardized mean difference (SMD) with 95 % CIs when different scales were used to measure the same outcome. The statistical heterogeneity among different studies was measured by calculating the I2 index. An I2 greater than 50 % was considered moderate heterogeneity. When p was < 0.05, it was defined as statistically significant.
RESULTS
CHARACTERISTICS OF THE INCLUDED STUDIES
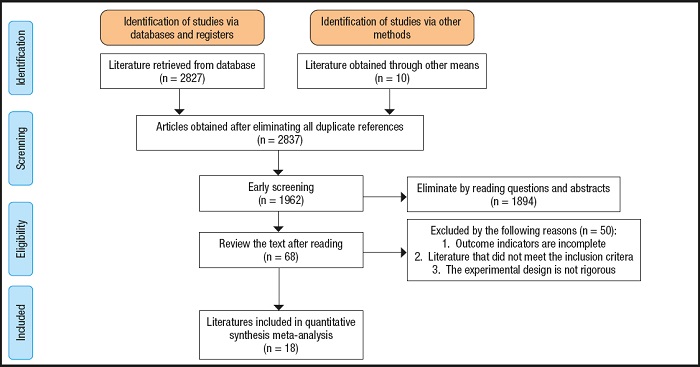
The initial search resulted in 2,837 potentially relevant articles. Our search identified 1,962 publications after eliminating duplicates. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart is presented in figure 1. The assessment of the risk of bias in the included trials is presented in figure 2A and figure 2B. After our initial screening by titles and abstracts, 68 records were excluded. After inspecting the full text, 18 RCTs were included. The characteristics of the included RCTs (16-33) are listed in table I.
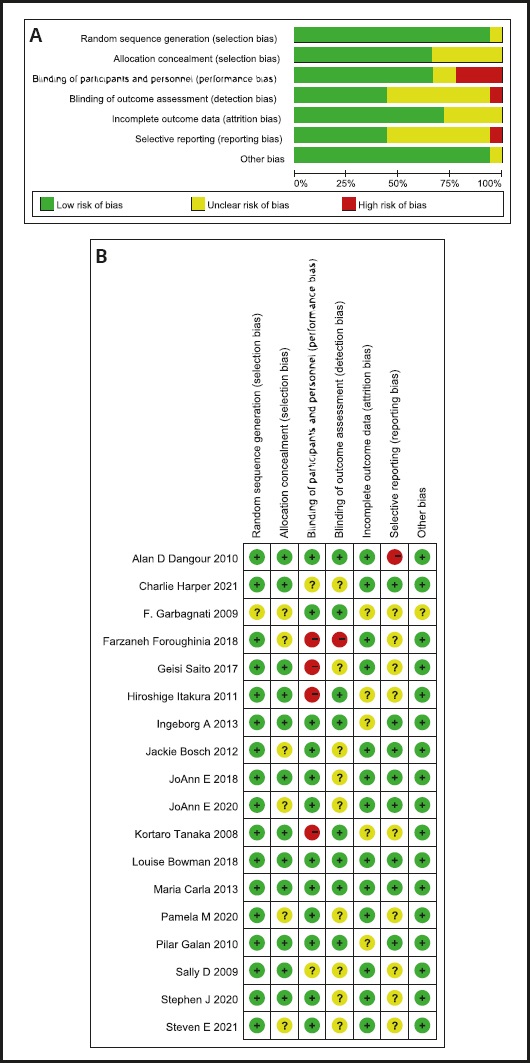
Figure 2 A. Risk of bias assessment for included trials. B. Risk of bias assessment of included trials.
Table I. Characteristics of the included RCTs.
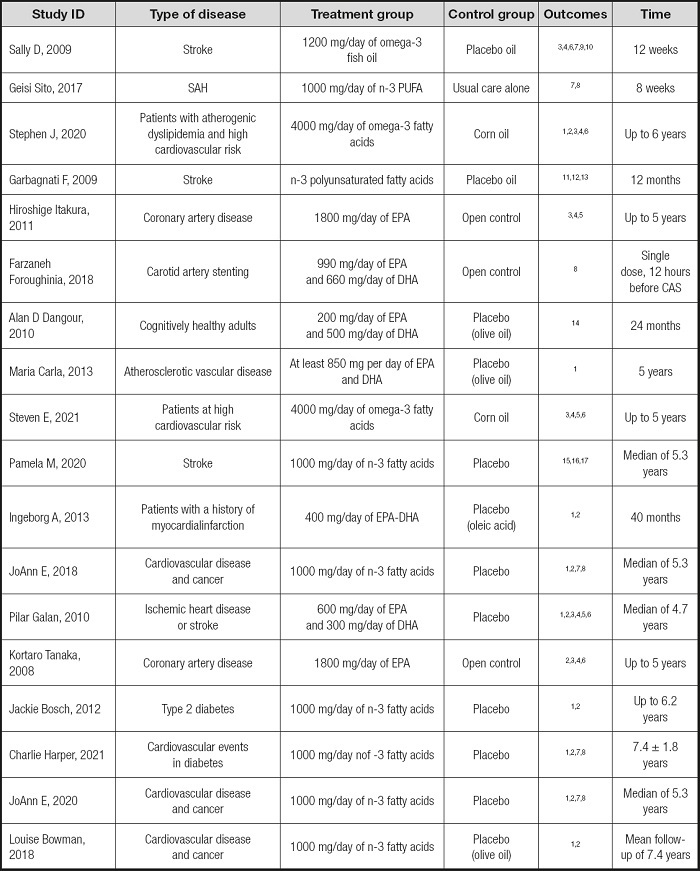
1: death;
2: stroke;
3: LDL;
4: HDL;
5: triglyceride;
6: total cholesterol;
7: ischemic stroke;
8: hemorrhagic stroke;
9: SF-36 questionnaire;
10: General Health Questionnaire—28-item;
11: CNS;
12: BI;
13: RMI;
14: CVLT (California Verbal Learning Test);
15: Nagi Scale;
16: Rosow-Breslau Scale;
17: Katz ADL scale.
EFFECT OF OMEGA-3 POLYUNSATURATED FATTY ACIDS ON VASCULAR DISEASE-RELATED DEATH
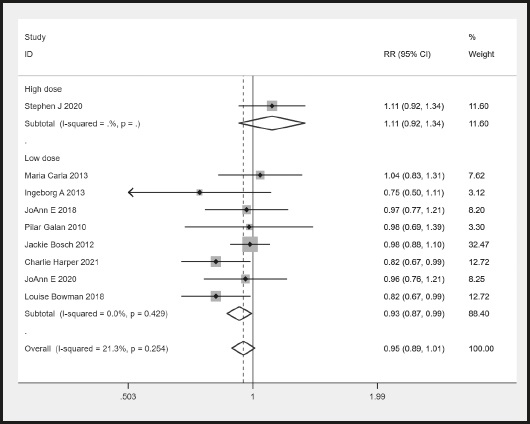
There were nine RCTs that provided the number of participants who had a vascular disease-related death. There was no significant heterogeneity (I2 = 21.3 % < 50 %). The meta-analysis showed no difference in the rate of vascular disease-related death between the n-3 PUFA and control groups (RR, 0.95, 95 % CI: 0.89 to 1.01, p = 0.114 > 0.05) (Fig. 3). However, there was a significant difference in the lower 3 PUFA dose subgroup (RR, 0.93, 95 % CI: 0.87 to 0.99, p = 0.034 < 0.05) (Fig. 3).
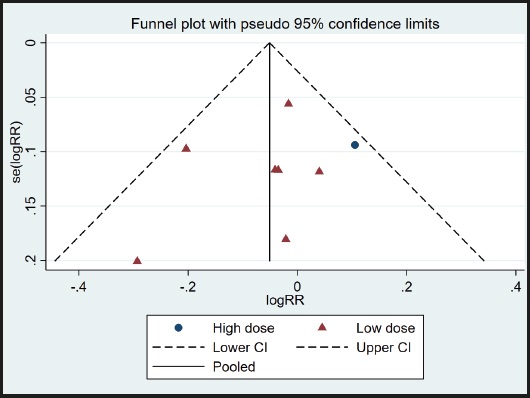
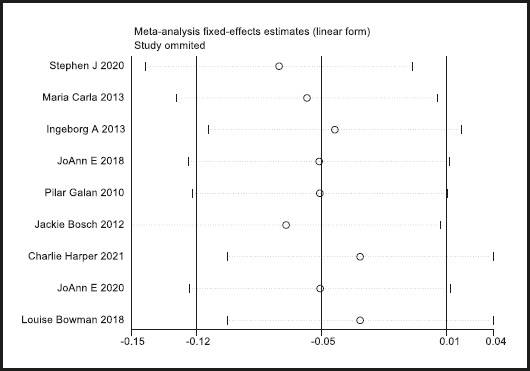
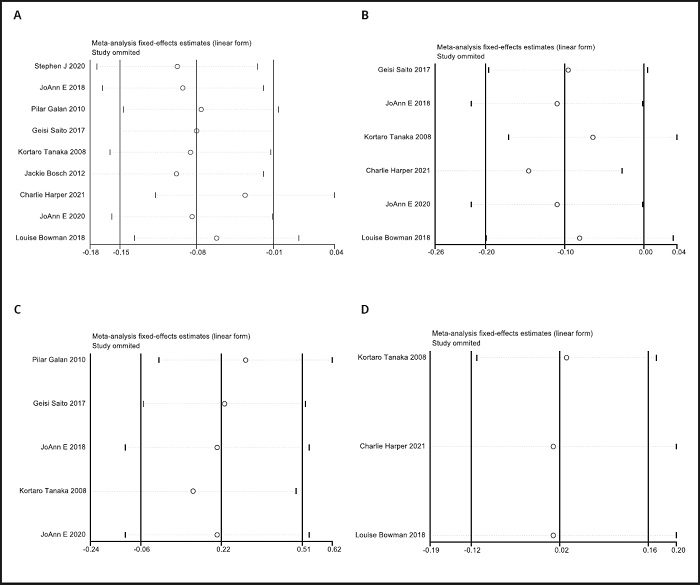
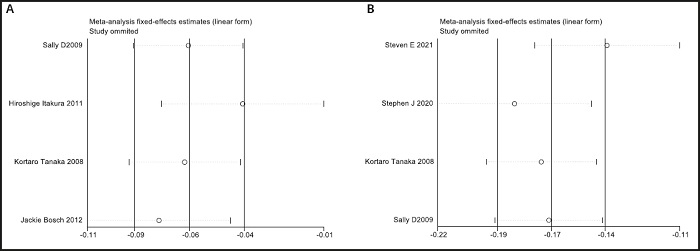
A funnel plot was used to evaluate publication bias. As shown in figure 4, the plot was basically symmetrical. Furthermore, Egger's test and Begg's test were performed to assess asymmetry, and it was concluded that the p-value of Egger's test was 0.485 > 0.05, while the p-value of Begg's test was 0.917 > 0.05. Therefore, it was believed that no publication bias was present in the articles included in this study. To ensure the accuracy and stability of the research, we further conducted a sensitivity analysis. The results showed that none of the articles caused great interference with the results of this meta-analysis, which means that the research has good stability (Fig. 5).
EFFECTS OF OMEGA-3 POLYUNSATURATED FATTY ACIDS ON CEREBROVASCULAR EVENTS
There were 9, 6, 5 and 4 RCTs that reported stroke, ischemic stroke, hemorrhagic stroke and transient ischemic attack (TIA), respectively. No heterogeneity was observed across the studies regarding stroke (I2 = 0 %), ischemic stroke (I2 = 0 %), hemorrhagic stroke (I2 = 0 %) and TIA (I2 = 0 %). The meta-analysis demonstrated that n-3 PUFAs did not significantly reduce the incidence of cerebrovascular events. The pooled RR was 1.00 (95 % CI: 0.93 to 1.07) for stroke (Fig. 6A), 0.99 (95 % CI: 0.896 to 1.094) for ischemic stroke (Fig. 6B), 1.249 (95 % CI: 0.939 to 1.662) for hemorrhagic stroke (Fig. 6C) and 1.016 (95 % CI: 0.882 to 1.170) for TIA (Fig. 6D).
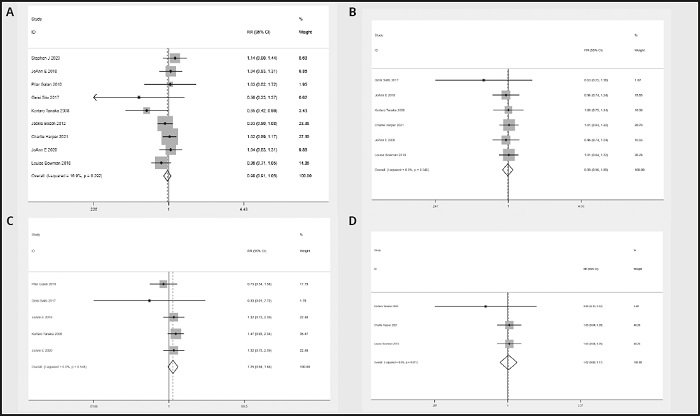
Figure 6 A. Forest plot of the difference in stroke between n-3 PUFAs and placebo. B. Forest plot of the difference in ischemic stroke between n-3 PUFAs and placebo. C. Forest plot of the difference in hemorrhagic stroke between n-3 PUFAs and placebo. D. Forest plot of the difference in TIA between n-3 PUFAs and placebo.
Funnel plots were used to evaluate publication bias. As shown in figure 6A to figure 7D, the plots were basically symmetrical. Furthermore, Egger's tests and Begg's tests were performed to assess asymmetry, and it was concluded that the p-values of Egger's tests and Begg's tests were > 0.05. Therefore, it was believed that there was no publication bias in the articles included in this study. The results showed that none of the articles caused great interference with the results of this meta-analysis, which means that the research has good stability (Fig. 8A to Fig. 8D).
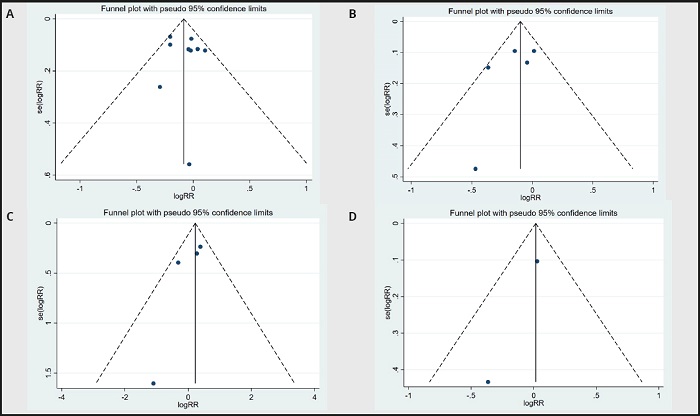
Figure 7 A. Funnel plot of stroke between n-3 PUFAs and placebo. B. Funnel plot of ischemic stroke between n-3 PUFAs and placebo. C. Funnel plot of hemorrhagic stroke between n-3 PUFAs and placebo. D. Funnel plot of TIA between n-3 PUFAs and placebo.
EFFECT OF OMEGA-3 POLYUNSATURATED FATTY ACIDS ON SERUM LIPID PROFILES
There were 6 RCTs that provided data about LDL and HDL levels. Regarding the LDL and HDL domains, no heterogeneity was present among these studies (LDL: I2 = 0 %; HDL: I2 = 0 %). The meta-analysis demonstrated a remarkable increase in the LDL levels in the n-3 PUFA group, with an SMD of 0.022 (95 % CI: 0.005 to 0.040) (Fig. 9A). The meta-analysis showed no difference in HDL levels in the n-3 PUFA group, with an SMD of 0.008 (95 % CI: -0.009 to 0.025) (Fig. 9B).

Figure 9 A. Forest plot of the difference in LDL between n-3 PUFAs and placebo. B. Forest plot of the difference in HDL between n-3 PUFAs and placebo.
Funnel plots were used to evaluate publication bias. As shown in figure 10A and figure 10B, the plots were basically symmetrical. Furthermore, Egger's tests and Begg's tests were performed to assess asymmetry, and it was concluded that the p-values of Egger's tests were > 0.05 (LDL: p = 0.517; HDL: p = 0.417), and the p-values of Begg's tests were > 0.05 (LDL: p = 0.348; HDL: p = 0.573). Therefore, it was believed that there was no publication bias in the articles of this study. The results showed that none of the articles caused great interference with the results of this meta-analysis, which means that the research has good stability (Fig. 11A and Fig. 11B).
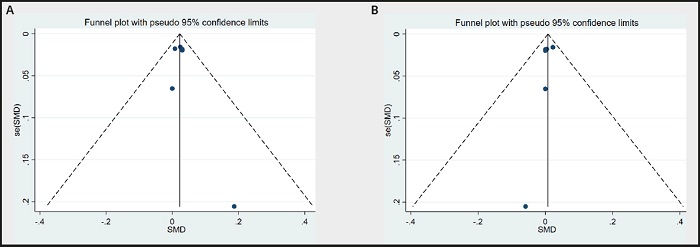
Figure 10 A. Funnel plot of LDL between n-3 PUFAs and placebo. B. Funnel plot of HDL between n-3 PUFAs and placebo.
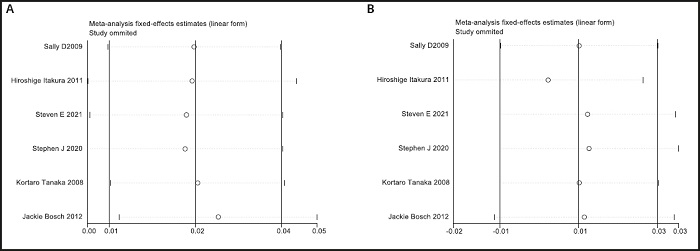
Figure 11 A.Sensitivity analysis of LDL between n-3 PUFAs and placebo. B. Sensitivity analysis of HDL between n-3 PUFAs and placebo.
There were 4 RCTs that provided data about TG and TC levels. Regarding the TG domains, no heterogeneity was present among these studies (I2 = 23.6 %). There was significant heterogeneity across the trials in terms of TC levels (I2 = 66.6 %). The meta-analysis showed a remarkable decrease in TG levels (SMD, -0.065, 95 % CI: -0.087 to -0.042, p = 0.00 < 0.05) (Fig. 12A) and TC levels (SMD, -0.167, 95 % CI: -0.193 to -0.141, p = 0.00 < 0.05) (Fig. 12B) in the n-3 PUFA group.
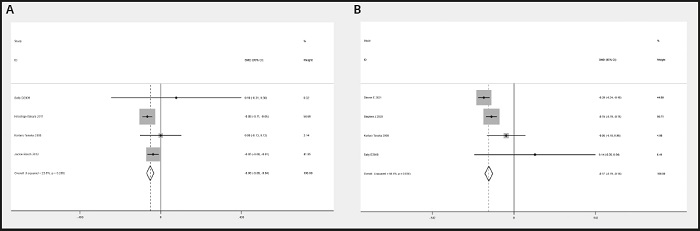
Figure 12 A. Forest plot of the difference in TG between n-3 PUFAs and placebo. B. Forest plot of the difference in TC between n-3 PUFAs and placebo.
Funnel plots were used to evaluate publication bias. As shown in figure 13A to figure 13B, the plots were basically symmetrical. Furthermore, Egger's tests and Begg's tests were performed to assess asymmetry, and it was concluded that the p-values of Egger's tests were > 0.05 (TG: p = 0.311; TC: p = 0.497), and the p- values of Begg's tests were > 0.05 (TG: p = 1.0; TC: p = 0.258). Therefore, it was believed that there was no publication bias in the articles of this study. The results showed that none of the articles caused great interference with the results of this meta-analysis, which means that the research has good stability (Fig. 14A and Fig. 14B).
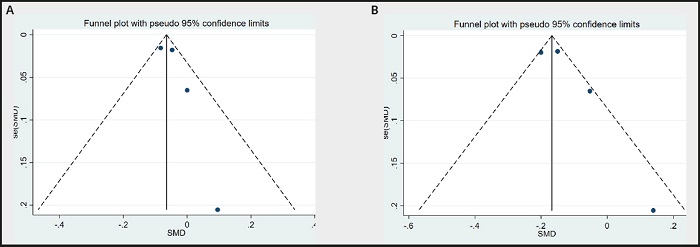
Figure 13 A. Funnel plot of TG between n-3 PUFAs and placebo. B. Funnel plot of TC between n-3 PUFAs and placebo.
DISCUSSION
This study is a systematic review and meta-analysis of randomized controlled trials that evaluated the use of n-3 PUFAs for stroke prevention and treatment. Through years of follow-up, the rate of vascular disease-related deaths did not decrease significantly after n-3 PUFA treatment compared with placebo treatment (RR, 0.95, 95 % CI: 0.89 to 1.01), which is consistent with the conclusion drawn by CD (34). However, after subgroup analysis, the rate of vascular disease-related deaths in the low-dose n-3 PUFA group was significantly lower than that in the control group (RR, 0.93, 95 % CI: 0.87 to 0.99). The results of a randomized trial that used higher doses of n-3 PUFAs showed no significant reduction in vascular disease-related deaths in the experimental group (18). Different doses of n-3 PUFAs produce different results in different regions (35); this was shown in studies such as JELIS (36) and REDUCE-IT (38), which tended to use high doses of n-3 PUFAs.
Based on some animal experiments, n-3 PUFAs have been confirmed to improve nerve damage, promote collateral circulation and improve nerve function after stroke (37-40). However, in this study, there was no significant difference in the total number of strokes, ischemic strokes, hemorrhagic strokes or TIAs between the two groups.
The treatment of stroke patients with n-3 PUFAs does not seem to reduce the risk of cardiovascular and cerebrovascular accidents. In addition, in 2 RCTs the incidence of cerebrovascular accidents in the n-3 PUFA group was significantly different from that in the placebo group, and in the other 7 RCTs, the number of cerebrovascular accidents in the n-3 PUFA group was not significantly improved.
n-3 PUFAs act on hepatocytes, increase the activity of protein enzymes, promote the clearance of very low-density lipoprotein in surrounding tissues, and reduce the levels of triglycerides, cholesterol and low-density lipoproteins in the serum. The lipid data revealed a significant improvement in TC and TG levels, which is consistent with a recent meta-analysis (41). Surprisingly, n-3 PUFAs did not exert a significant beneficial effect on LDL or HDL levels. Statins exert both synergistic and antagonistic effects with n-3 PUFAs (42), and it is unclear whether these effects are related to statin-based therapy in some studies.
In general, there is a certain degree of heterogeneity in this study (TG and TC levels showed moderate heterogeneity), and this heterogeneity is considered to be related to the different dietary habits of people in different regions, the amount of n-3 used for supplementation, and the age and sex of the patients.
In recent years, the physiological activity of n-3 PUFAs has been a research hotspot in the fields of food and medicine, and n-3 PUFAs have been proven to have positive effects on the prevention and treatment of diabetes, and of cardiovascular and cerebrovascular diseases, as well as to exert anticancer, anti-inflammatory, and hypolipidemic effects (7,8,43-44). N-3 PUFAs also improve neurological impairment after stroke, especially in some malnourished patients (19,26). There is increasing evidence that ceramides are closely related to atherosclerosis (45-46), and n-3 PUFAs can reduce plasma ceramide levels to reduce the occurrence of cardiovascular and cerebrovascular accidents (47-49). N-3 PUFAs can reduce the volume of cerebral infarction to a certain extent by regulating the activity of antioxidant enzymes (50). N-3 PUFAs inhibit brain cell inflammatory mechanisms by increasing the expression of Nrf2 and HO-1 (51), resulting in neuroprotection. Oral n-3 PUFA supplementation not only improves hemorrhagic stroke outcomes (52) but also reduces the risk of complications (53). The optimal dose of n-3 is still controversial, and patients with atrial fibrillation do not benefit from high doses of n-3 PUFAs (38), so the lowest dose of n-3 PUFAs should be used to achieve maximum efficacy.
LIMITATIONS
There are still some limitations:
- The number of studies included in this study was small, and the quality of the literature was not high.
- The amount of n-3 used for supplementation in each study was not unified, and there were different treatment times.
- At present, there are few studies on the treatment of stroke with n-3 PUFAs in various countries, so the results cannot be further meta-analyzed and further verified.
CONCLUSIONS
Current evidence suggests that supplementation with n-3 PUFAs can reduce cardiovascular and cerebrovascular disease-related death and reduce TC and TG levels, but it cannot prevent the occurrence of cerebrovascular accidents or improve LDL or HDL levels. We look forward to future randomized controlled trials with higher quality, larger populations, and longer follow-up times to overcome the shortcomings of this study.