INTRODUCTION
Food addiction is defined as an uncontrolled craving for food and anxiety about food. It has been shown that dopamine release is stimulated in the brain, especially following carbohydrate intake, which is similar to the effects of substances such as nicotine and alcohol (1). The symptoms of food addiction that predispose individuals to develop obesity are prevented by a diet rich in antioxidants, and the feeling of hunger and food intakes are reduced (2); such diets are also protective against high oxidative stress as caused by diets with high fat and sugar contents (3).
Oxidative stress is a risk factor for obesity, both in itself and as a mediator in metabolic processes. It also plays a role in adipogenesis and is associated with leptin resistance, chronic inflammation, sleep disturbance, dysbiosis, insulin resistance, adipocytokines, and obesity genes (4). It also plays a role in the food addiction behaviors of obese and overweight individuals by acting on brain signals and neurotransmitters (5).
In studies, it was shown that zinc (Zn) is an effective antioxidant to decrease oxidative stress, and there was a relationship between high oxidative stress and Zn redistributions in plasma, urine, erythrocytes and saliva (6). Low Zn levels were detected in obese individuals in many studies (7). On the other hand, studies show a correlation between appetite and serum zinc levels that leads to obesity (8).
Food addiction develops in a neurobiological, behavioral, and clinical framework that is parallel to substance addiction, and the genetic background plays an important role in addition to environmental factors (9). Dopamine, which is associated with addiction, is the main neurotransmitter involved in the reward system in the brain (10). With food intake, dopamine release from the dorsal striatum begins, and it has been reported that the degree of pleasure derived from eating is proportional to the amount of dopamine released (11). It has also been suggested that the numbers of dopamine receptor 2 (DRD2) in the central nervous system are lower in obese individuals than in nonobese individuals, and they have a greater desire to eat to compensate for this situation (12). In studies, it has been determined that the DRD2 receptor gene Taq1A (rs 1800497) and toll-interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP rs625413) gene polymorphisms are responsible for increases in food addiction, especially for carbohydrate foods (1,10).
Physical activity prevents the risk of obesity development by helping maintain energy balance (13). Additionally, it was noted that energy intake, physical activity and DRD2 Taq1A gene polymorphisms prevent overweight and obesity (14).
Although there is a study evaluate the relationships among oxidative stress, food addiction, physical activity and obesity (15), no study exists that has evaluated the relationship between food addiction and gene polymorphisms in recreationally active individuals.
In this study, we hypothesized that food addiction is associated with genetic polymorphisms, decreased serum total antioxidant capacities and Zn levels. We aimed to question the possible effect of Zn on appetite in food addiction separately from its antioxidant properties. Recreationally active women were selected, and the relationships among DRD2/TIRAP gene polymorphisms, food addiction, antioxidant capacities, and zinc were evaluated among those who were engaged in regular activities at least 3 days per week. There is only one study in the literature that evaluates the relationship between gene polymorphisms and antioxidant levels (16); therefore, our study will also contribute to this subject.
MATERIALS AND METHODS
PARTICIPANTS
This cross-sectional-analytical study was conducted among women who were engaged in recreational activities. To conduct this study, approval by the ethics committee (decision number 2019/17-42 and dated 02/07/2019) was obtained from the Hacettepe University Non-Interventional Clinical Research Ethics Committee. The aims and methods of the study were explained to the participants, and those who agreed to participate in the study were asked to sign a voluntary consent form that followed the Declaration of Helsinki (World Medical Association).
In this context, 210 young adult women (18-31 years old) were enrolled between December 2020 and April 2021 at a university in Ankara (95 % power), and the analysis process included 156 women who fully complied with the study and did not have any deficiencies/misrepresentations in their data. The inclusion criteria included the following: age greater than 18 years, engaging in recreational activities (at least 3 days of regular exercise per week), not diagnosed with depression by a doctor, not using antidepressants or oral contraceptives, not diagnosed with cancer, liver or kidney failure, without overt diabetes, consenting to participate in the study, and researchers' decisions.
DETERMINATION OF GENERAL CHARACTERISTICS
Within the scope of the study, a questionnaire form to evaluate the general information and health histories of the participants was used by the researchers by using a face-to-face interview technique.
EVALUATION OF FOOD ADDICTION
The food addiction status of participants was evaluated using the "Yale Food Addiction Scale." The Yale Food Addiction Scale was developed by " et al. (2009) (17) to obtain the eating habits of the participants during the previous year based on the seven symptoms and eating disorders specified in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (18).
A Turkish validity and reliability study of this scale was carried out by Bayraktar et al. in 2012 (19). The scale consists of 26 items, and the answers provided to the questions are scored 0 or 1 point. The score for each diagnostic criterion was calculated separately. The total score varied between 0 and 7. To determine food addiction, the 15th or 16th question is important, and 1 point should be taken. When the number of symptoms is more than 3, a diagnosis of food addiction is made.
ANTHROPOMETRIC MEASUREMENTS
Body weights (kg) were measured by bioelectrical impedance analysis (BIA) using a TANITA BC418-MA Segmental Body Composition Analyzer (Tokyo, Japan) when the participants were hungry in the morning and wearing light clothes. Height measurement (cm) were conducted using a stadiometer with standard protocols. BMIs were calculated as "body weight/height2" (kg/m2), and the classification of the World Health Organization (WHO) according to BMI in adults was used (20).
BIOCHEMICAL ANALYSIS
Venous blood samples were obtained from participants in the morning after 8 h of fasting. Serum samples were stored in a deep freezer at −80 °C until analysis. Total antioxidant capacity analysis was performed with an enzyme-linked immunosorbent assay using a commercial kit (Relassay, Turkey). In this method, the antioxidants in the samples convert the dark blue-green ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical solution into a colorless ABTS form. Absorbance changes at a wavelength of 660 nm are related to the total amounts of antioxidants. The results are expressed in vitamin E-like Trolox equivalents (mmol Trolox equivalent/L) (21).
Zinc present in the samples changes the red-orange color of 5-Br-PAPS to light pink under alkaline conditions. The absorbance changes at 548 nm are proportional to the total zinc levels (μg/dL) in samples. The test can be calibrated with zinc sulfate dissolved in deionized water (Relassay, Turkey).
GENETIC ANALYSIS
DNA extraction was performed with the isolation kit, GF-1 Blood DNA Extraction Kit (Vivantis, Malaysia), for whole blood samples collected in EDTA tubes.
The nucleic acid loads (ng values) of the samples obtained from the total DNA extraction process were measured using a Colibri Microvolume Spectrometer (Titertek-Berthold, Germany) device for use in the next steps of the study.
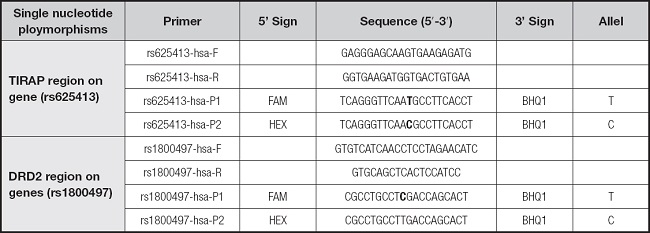
Within the scope of this study, the oligonucleotide designs of single-nucleotide polymorphisms with known nucleotide database codes for gene regions were studied with a real-time polymerase chain reaction (PCR) device (Table I).
Table I. Presentation of oligonucleotide regions on toll-interleukin 1 receptor (TIR) domain- containing adaptor protein (TIRAP rs625413) and dopamine receptor 2 (DRD2) genes.
The lyophilized primers synthesized in the study were dissolved with nuclease-free dH2O to a 100 mM concentration. For real-time PCR, a SensiFAST Probe No-ROX Kit (Bioline, US) was used. For a 20-μl reaction mix, 10 μL of SensiFAST Probe Mix (1X), 2.5 μL of Primer/Probe mix (0.3 mM forward primer 10 mM) and 0.3 mM reverse primer (10 mM), 0.1 mM probe (P1-P2, 10 mM), 2.5 μl of DNA, and 5 μl of nuclease-free dH2O were used. The reaction was performed using LightCycler 480 (Roche, Switzerland) 8-strip tubes with the following program: initial denaturation at 95 °C for 5 min, 40 repetitions at 95 °C for 10 sec, 66 °C (rs625413), 68 °C (rs1800497) for 30 sec (read), and fragment duplication. Real-time PCR was based on the allele-specific probe data obtained (Table II).
Table II. List of primer-probe sets used for toll-interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP rs625413) and dopamine receptor 2 (DRD2) genes region detection.
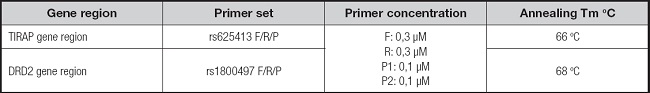
F: forward primer; R: reverse primer; P: probe.
To detect polymorphisms in the DRD2 gene region (rs1800497 T > C), fluorescent dye-labeled probes for the C (FAM) and T (HEX) alleles were used. Using real-time PCR, the genotypes were determined as CC (wild-type genotype), TT (homozygous genotype for the risk allele), and CT (heterozygous genotype for the risk allele) according to the fluorescence levels.
Fluorescent dye-labeled probes for the T (FAM) and C (HEX) alleles were used to detect polymorphisms in the TIRAP gene region (rs625413 T > C). By using real-time PCR, the genotypes were determined as TT (homozygous genotype for the risk allele), CC (wild type genotype), and TC (heterozygous genotype for the risk allele) according to the fluorescence levels.
The frequencies of the alleles detected as a result of genetic analysis were evaluated according to the Hardy-Weinberg balance (p + q = 1), and genotype frequencies were compared with expected frequencies according to Hardy-Weinberg (22).
Hardy-Weinberg equation = p2 + 2pq + q2 = 1.
For DRD2 Taq 1A genotype frequencies:
STATISTICAL ANALYSES
Data obtained in the study were evaluated with Statistical Package for the Social Sciences (SPSS) 22.0 software. The normality of the data distribution was examined using visual (e.g., histogram and probability graphs) and analytical methods (e.g., Kolmogorov-Smirnov/Shapiro-Wilk tests). The χ2 test was used to compare general characteristics according to food addiction status and gene variants. For comparing serum total antioxidant capacities and zinc levels of the participants in this study in terms of food addiction status (presence/absent), independent sample tests (t or z tests) or Mann-Whitney U-tests were used. In the comparisons of serum antioxidant capacities and zinc levels of individuals according to gene variants, a one-way analysis of variance or the Kruskal-Wallis test was used. Spearman's and Pearson's correlation tests were used to evaluate the relationships among different parameters. In all analyses, a value of α = 0.05 was chosen as the error level, and it was interpreted that "the difference is statistically meaningful/significant" for p-values equal to or less than this value.
RESULTS
A total of 156 volunteer women with a mean age of 20.47 ± 2.00 years and BMI of 24.55 ± 3.05 kg/m2 were included in this study, and 12.3 % of them were underweight, 41.4 % were of normal weight, 36.7 % were overweight, and 9.6 % were obese.
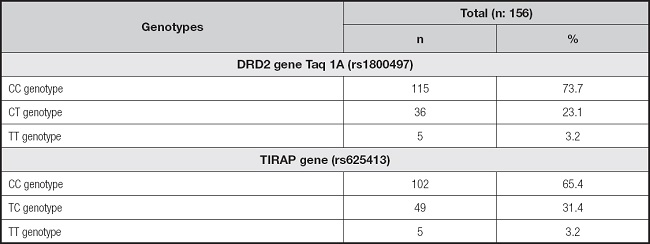
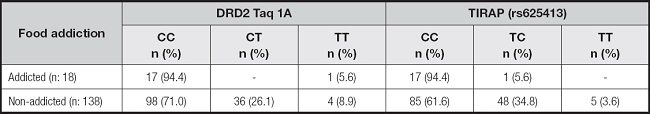
The distributions in the frequencies of the DRD2 Taq 1A (rs1800497 T > C) and TIRAP gene (rs625413) polymorphisms of participants are shown in table III. The frequencies of participants with DRD2 Taq 1A and TIRAP homozygous genotypes (homozygous for the risk allele) were determined to be 3.2 %, and those with heterozygous genotypes were 23.1 % and 31.4 %, respectively (Table III).
Table III. The frequencies of dopamine receptor 2 (DRD2) Taq 1A (rs1800497) and TIRAP (rs625413) genotypes of recreationally active Turkish women.
DRD2 Taq 1A (rs1800497 T > C) (CC: 72.6 %; CT: 25.1 %; TT: 2.1 %) and TIRAP (CC: 65.7 %; TC: 30.6 %; TT: 3.5 %) genotype frequencies were not different from those expected from Hardy-Weinberg equilibrium (p > 0.05).
In addition, it was determined that most participants had nonpolymorphic/wild genotypes (CC genotype) in the DRD2 and TIRAP genes (Table III).
It was determined that 11.5 % of the participants had food addiction. The distributions of the DRD2 Taq 1A (rs1800497) and TIRAP (rs625413) genotype frequencies according to the food addiction status of the participants are shown in table IV. Accordingly, 94.4 % of participants with and without food addiction had the DRD2 Taq 1A CC genotype and TIRAP gene CC genotype (nonpolymorphic/wild genotype). Homozygous/at-risk genotypes in terms of risk alleles were not detected in the DRD2 Taq 1A and TIRAP genes of participants with food addiction (Table IV).
Table IV. The frequencies of dopamine receptor 2 (DRD2) Taq 1A (rs1800497) and TIRAP (rs625413) genotypes according to the food addiction status of recreationally active Turkish women.
The serum antioxidant capacities and Zn levels of the participants with different genotypes and food addiction statuses are compared in table V. There were no statistically significant differences between the serum antioxidant capacities and zinc levels of participants with and without food addiction who had different DRD2 Taq 1A genotypes (p > 0.05). Similarly, there were no differences between the serum antioxidant and zinc levels of women with and without food addiction with different TIRAP (rs625413) genotypes (p > 0.05) (Table V).
Table V. Serum antioxidant capacities (mmol Trolox equivalent/L) and zinc levels (μg/dL) of the recreationally active Turkish women according to their food addiction status and DRD2 Taq 1A (rs1800497) and TIRAP (rs625413) genotypes.
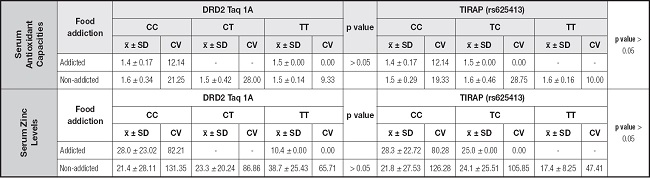
x̅: mean; SD: standard deviation; CV: coefficient variation.
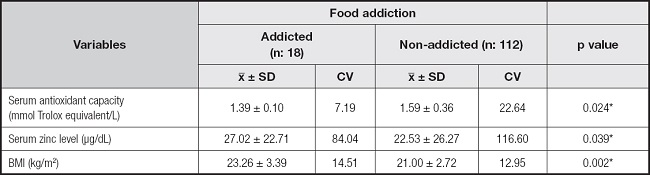
Serum antioxidant capacities, zinc levels and BMI of the recreationally active Turkish women according to their food addiction status are shown in table VI. There were statistically significant differences between the serum antioxidant capacities, serum zinc levels and BMI of participants with and without food addiction (p < 0.05) (Table VI).
DISCUSSION
The food addiction hypothesis suggests that exposure to palatable foods alters the brain's reward circuit and drives the behavioral phenotype to compulsive overeating (23). Food addiction is related to overeating outcomes of obesity and micronutrient deficiencies (24). Recently, some data have shown that genetic polymorphisms may be associated with food addiction (14). In this study, our hypothesis was rejected; the DRD2/TIRAP genotype was not found to be related to food addiction, serum total antioxidant capacities, or antioxidant and appetite stimulant agent Zn levels among recreationally active Turkish women.
In a study, the relationship between food addiction and physical activity was evaluated in university students, and more food addiction symptoms were detected in association with high physical activity levels (25). In another study that evaluated 1344 adults, lower physical activity levels (less than 1.8 hours of walking per week, less than 32 minutes of walking per week, or less than 58 minutes of normal or vigorous exercise per week) in individuals with food addiction were determined to be inversely related. It has been shown that the frequency and duration of physical activity may be inversely related to food addiction (26). In this study, it was ensured that all the participants were recreationally active, which made it possible to exclude the effect of different physical activity levels on food addiction and observe the effect of only genetic polymorphisms on food addiction.
Food addiction levels were determined to be 11.5 % in our study. In another study conducted in university students in Turkey, it was found to be 9.0 % (27). The difference in our findings can be explained by the fact that all of our samples consisted of women, and food addiction is higher in women than in men. In a study that evaluated food addiction in 967 university students in the US, the incidence of food addiction was found to be higher in women than men (12.3 % vs. 4.6 %) (28). In other study in which food addiction was evaluated in university students in Spain, it was shown that the rate of food addiction was 6.4 % (26).
DRD2 Taq 1A was the first genetic polymorphism identified as responsible for food addiction (15). In this study, it was determined that the food addiction frequency in the participants with DRD2 Taq 1A homozygous genotypes (homozygous for the risk allele) was 3.2 %, and the frequencies of those with heterozygous genotypes were 23.1 % and 31.4 %. In Asian students, CT variants were found to be higher than TT and CC of the DRD2 Taq1A gene polymorphism (29). It has been reported that the frequency of the DRD2 Taq 1A genetic polymorphism is 2.95 % (30) in Caucasians, 37.9 % and 29 % (31) in China and India. Our findings are compatible with those of the Caucasian population. The fact that the origin of Caucasian cuisine comes from Circassian cuisine and that the characteristics of this culture are seen in the Aegean Region in our country explain the similarity. Ethnicity and diet can influence differences between genotypes and alleles.
In a Mexican study, the DRD2 TaqIA polymorphism was shown to be associated with an increased frequency of unhealthy food consumption (32). In our study, no risky genotype was found in terms of the risk alleles in the DRD2 Taq 1A gene of participants with food addiction. Additionally, in our country, similar evidence was found to evaluate the association among DRD2 Taq 1A and 1B polymorphisms and other addiction conditions, namely, heroin, which did not show any difference in terms of this genetic polymorphism between individuals (33). It has been stated that this relationship may vary among populations due to differences in the frequency of the TaqIA allele (32).
The second gene evaluated concerning food addiction status is TIRAP. It is involved in cytokine release, the inflammatory response, and the regulation of TNF-α production (33). In a study evaluating food addiction in overweight/obese individuals, lower TNF-α levels were found in the obese group, and it has been stated that low levels of TNF-α, an anorexigenic cytokine, may be related to TIRAP gene polymorphisms (34).
Of these data, TIRAP is a newly discovered single nucleotide ploymorphisms (SNP) region associated with addiction, and there are not enough relevant studies in the literature. We did not find any association between TIRAP and food addiction in our study.
Food addiction occurs during the consumption of frequently processed foods, and these foods have no or extremely low micronutrient and antioxidant contents (24). Therefore, we investigated the relationships among serum total antioxidant capacities, Zn levels, and food addiction in our study. Since the definition of total antioxidant capacity covers the cumulative effect of all antioxidants and provides more accurate results than separate evaluations (35), we preferred to evaluate the total serum antioxidant capacity.
In the present study, although no differences were found in the serum total antioxidant capacities and zinc levels based on genotypes, the serum antioxidant capacities were lower and Zn levels were higher in the group with food addiction.
For the serum Zn levels in individuals with food addiction, in a study evaluating the effect of food addiction on food consumption in our country, similar to our findings, the addiction status incidence was found to be 11.4 %, and zinc consumption was found to be higher in the addiction group (36). Considering the function of zinc in the sense of taste (37), it can be related to elevated serum levels in the food addiction group, which are known to be associated with high taste intensities, fat, sugar, and salt contents and excessive consumption of foods. Moreover, it is a micronutrient that can affect many neurotransmitters in the serotonergic, glutamatergic, and dopaminergic systems (38); therefore, in this study, we thought it would be related to the development of addiction-like behavior.
The tendency to consume prooxidant foods in individuals with food addiction promotes the production of antioxidant enzymes and reactive oxygen species that reveal the activation of heat (39). To protect health and prevent the oxidative stress imbalance detected in obesity and food addiction, it is recommended to consume prooxidant and antioxidant foods at a ratio of 2:3 per meal (40). In this study, the serum total antioxidant capacity of individuals with food addiction was found to be lower. It is necessary to develop recommendations to support antioxidant consumption by evaluating the daily food consumption of individuals with food addiction.
To the best of our knowledge, the present study is the first to evaluate the relationship among genetic polymorphisms, serum total antioxidant capacities and Zn levels. Despite the accumulated literature on DRD2, we investigated the novel candidate gene, TIRAP, which is another strength of this study.
Despite these strengths, there are also some limitations. First, the food consumption of individuals has not been evaluated, and evaluating only one gender is one of the other limitations. Comparing nutritional patterns with biochemical findings in the larger sample with both sexes will strengthen the findings.
In conclusion, in our study, it was shown that DRD2 and TIRAP gene polymorphisms are not associated with food addiction, serum antioxidant capacities, or Zn levels, whereas individuals with food addiction have low serum antioxidant capacities and high serum Zn levels. Despite the antioxidant properties of Zn, Zn levels were found to be high regardless of the low antioxidant capacity levels.