INTRODUCTION
Cognitive impairment is defined as a progressive decline in memory, learning, spatial positioning, reasoning, judgment, and the evaluation of brain functions. It is an unstable cognitive state and early intervention can prevent progression to dementia (1). Prevalence of cognitive impairment is 10-20 % for adults aged ≥ 65 years (2,3). There is a 25 % probability that cognitive impairment will progress to dementia within one year, and 50 % within ten years (4,5). What is more, the incidence of dementia increases with increasing age and reaches 20-40 % in adults aged ≥ 85 years. Recently, the relationship between cognitive impairment and vitamin B vitamins has attracted extensive attention.
Vitamin B12, also known as cobalamin (Cbl), is the only water-soluble vitamin containing metallic element cobalt. Vitamin B12 is only synthesized by microorganisms, and dietary vitamin B12 is provided mainly from animal foods, such as meat, dairy, eggs, fish and B12 supplements (6). Therefore, insufficiency of intake (vegetarians, intestinal diseases, etc.), absorption defects (pernicious anemia, Imerslund Grasbeck syndrome), transport disorders (transcobalamin defects), and cell processing defects (Cbl A-F mutations) can lead to vitamin B12 deficiency (7). Prevalence of vitamin B12 deficiency is 5-40 % in people beyond 60 years of age, depending on the diagnostic criteria used (8-11). Vitamin B12 is very important for the hematological and nervous systems. In addition, vitamin B12 is also a vital biologically active coenzyme: methylcobalamin and adenosylcobalamin, which are the cofactors for homocysteine methyltransferase and methylmalonyl CoA mutase (7,12), are essential for maintaining homeostasis of homocystine (Hcy) and methylmalonic acid (MMA). Vitamin B12 plays an essential role in the synthesis of neurotransmitters and structural elements of neurons (13), thus, vitamin B12 deficiency is associated with cognitive impairment and other mental disorders (14-16).
Some studies have shown that early supplementation of B vitamins can effectively reduce total Hcy (tHcy) levels in the elderly with high tHcy levels, slow down the rate of brain atrophy, reduce the levels of inflammatory cytokines in human peripheral blood (17), and prevent conversion from cognitive impairment to dementia (18-20). However, other researchers found that vitamin B12 and folic acid supplements did not significantly reduce cognitive decline in people with cognitive impairment (21,22). More importantly, there are relatively few randomized controlled studies on the improvement of cognitive function by vitamin B12 supplementation alone, and the specific clinical prognosis evaluation is not conclusive.
Therefore, the aim of the present work is to determine the effect of vitamin B12 supplementation on neuropsychological function and disease progression in middle-aged and elderly Chinese patients with cognitive impairment.
MATERIAL AND METHODS
STUDY DESIGN
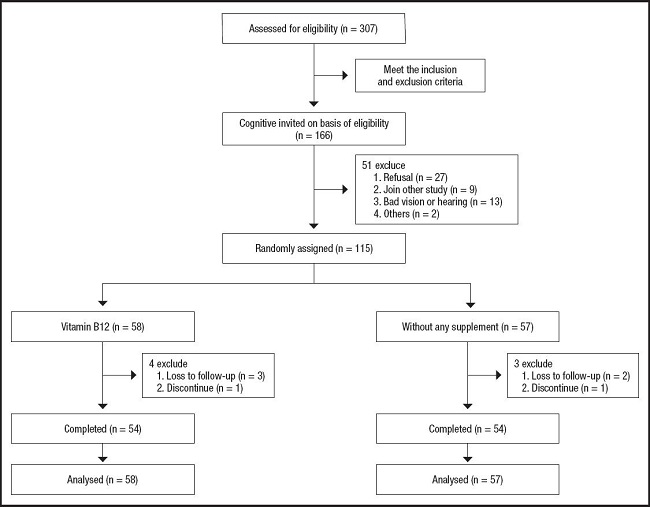
This was an interventional study to whether treatment with vitamin B12 slowed cognitive impairment progression in middle-aged and elderly Chinese patients. The participants were enrolled between May 2020 and May 2021. Trained graduate students and mental health clinicians performed relevant investigations. Details of the recruitment process are shown in figure 1.
SUBJECT DESCRIPTION
We prospectively collected data from participants diagnosed clinically with cognitive impairment and in stable condition from the Department of Neurology of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Inclusion criteria were as follow: a) age 45 years and over; b) a Mini-Mental State Examination (MMSE) score of less than 24, a Montreal Cognitive Assessment (MoCA) score of less than 26; c) willingness to participate in the study; and d) not using any nutritional supplementation known to interfere with nutrition status, folate metabolism, or cognitive function in the three months prior to recruitment.
Exclusion criteria were as follow: a) subjects diagnosed with bipolar disorder, Parkinson's disease, multiple sclerosis, motor neuron disease, a developmental disability, central nervous system inflammation, progressive malignancy, psychotic symptoms, or a diagnosis of schizophrenia or an alcohol or drug dependency; b) subjects were also excluded if they had any medical or psychological condition that prevented them from completing assessments; and c) incomplete patient clinical data and lack of cooperation with the investigators.
NUTRITIONAL INTERVENTIONS
Patients with cognitive impairment were randomly divided to receive vitamin B12 supplementation or control without any daily treatment for six months.
Injectable vitamin B12 was administered intramuscularly at a dose of 500 mg once a day for seven days, followed by cobamamide, which was given in the dose of 0.25 mg orally, and methylcobalamin in the dose of 0.50 mg orally every day during the next days. Both groups were closely followed up and monitored for six months and then again at six months using a repeat MMSE/MoCA score.
During the follow-up, both groups were closely monitored for any neurological or cognitive worsening. Approval from the Institutional Ethics Committee was obtained prior to the start of the study.
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki. All participants were informed of the study objectives, and their consent to participate in the study was obtained. The research protocol was approved by the Medical Ethics Committee of Chongqing Medical University, China.
DIAGNOSIS OF COGNITIVE IMPAIRMENT
The MMSE measures the general cognitive function. It comprises six domains: orientation, registration, attention and calculation, recall, language, and visuospatial function. The maximum scores for various domains ranged from 1 to 10. The six domain scores resulted in a total score of 30, with a higher score indicating better general cognitive function (23,24). The sensitivity and specificity of the MMSE have been examined in individuals with cognitive impairment (25).
The MoCA-30 is a brief cognitive function test. It comprises seven domains: short-term memory, visuospatial function, executive function, attention, concentration, working memory, language, and orientation. The implementation duration was approximately ten minutes (26). The maximum scores for the various domains ranged from 1 to 6. Together, the seven domain scores totaled 30, with higher scores indicating better general cognitive function. The sensitivity and specificity of the MoCA-30 have been examined in individuals with cognitive impairment (27,28).
BIOCHEMICAL ANALYSES
All patients underwent clinical history, neurologic examination, and complete blood work. Blood samples were collected at baseline and six months after venipuncture after a 10- to 12-hour overnight fast. Antecubital venous blood at 2-3 ml was collected from the patient on an empty stomach in the morning, centrifuged at 3,000 rpm for ten minutes, and analyzed by routine tests performed in the Department of Medical Laboratory of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
According to laboratory instructions, vitamin B12 deficiency was defined as a serum B12 concentration < 180 pg/ml. Folic acid deficiency (< 3 ng/ml), high levels of folic acid (> 19.9 ng/ml), and high levels of tHcy (> 15 µmol/l) were also defined. Hemoglobin (Hb) and mean corpuscular volume (MCV) levels were analyzed according to the age-adjusted normal ranges.
STATISTICAL ANALYSES
SPSS 22.0 software package was applied to perform the statistical analysis (IBM Corporation, version 22.0, for Windows). Frequencies and percentages were calculated. Continuous variables were examined using the Shapiro-Wilk first. If the data were normally distributed, the Student's t-test was used; otherwise, the non-parametric Mann-Whitney U test was used. The Chi-squared test (χ2 test) and Fisher's exact test were used for comparison between independent groups of categorical data. For all statistical tests, values of p < 0.05 (two-tailed) were considered as statistically significant.
RESULTS
A total of 307 patients were enrolled in the study from May 2020 to May 2021. A total of 192 patients were eventually excluded for the following reasons: 141 patients did not meet the inclusion and exclusion criteria, 27 patients refused to undergo cognitive testing, nine patients underwent another study, 13 patients suffered from bad vision or weak hearing, and two patients had other reasons. The recruitment, enrollment and flow of participants during the trial are shown in figure 1.
Finally, 115 patients were eligible for this study, 58 were randomly assigned to vitamin B12, and 57 to control group, respectively. Seven participants (2.3 %) were unable to complete the trial and the dropout rates were similar among all the groups (p > 0.05). On the basis of the number of unused capsules in the returned dispensers, the mean compliance was high, with 99 % of the capsules reportedly consumed.
Table I shows the baseline characteristics of the study population. No significant differences were observed between the two treatment groups (p > 0.05). The randomization procedure was successful because the groups were fairly well balanced in terms of demographic, biochemical, and cognitive data. No adverse events were reported during the trial.
Table I. Baseline characteristics of participants with cognitive impairment by treatment groups.
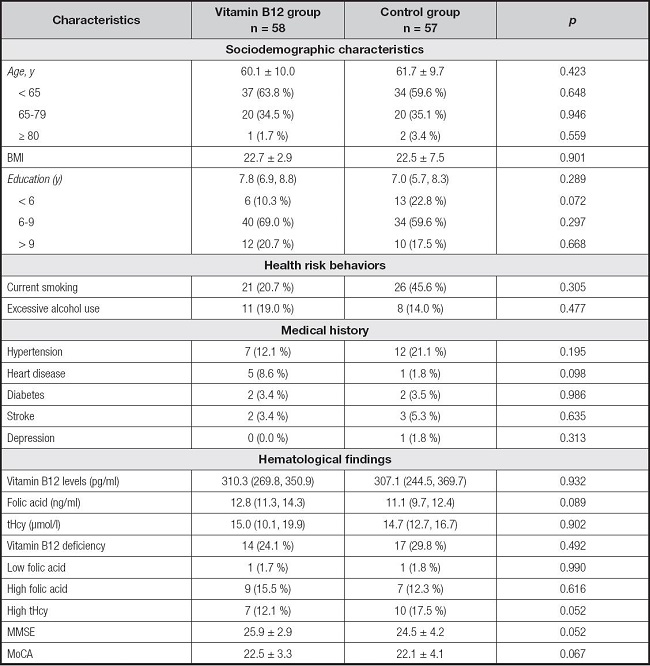
BMI: body mass index; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment.
Variables are presented as %, median (P25, P75) or mean ± SD.
The results are presented in tables II and III. At month 6, the vitamin B12 treatment group showed a significant increase in serum B12 levels (p < 0.01) and MMSE scores compared to those before treatment (p < 0.01). The vitamin B12 treatment group had an increase in MoCA score and a reduction in tHcy levels compared to those before treatment, but the difference was not significant (p > 0.05). Moreover, at month 6, the vitamin B12 treatment group showed a significant increase in serum active B12 (p < 0.01) and MMSE/MoCA scores compared to the control group (p < 0.01).
Table II. The levels of blood biomarker parameters at baseline and after sixth month of supplementation with vitamin B12 or control.

Variables are presented as median (P25, P75).
Compared with before treatment, *p < 0.05, †p < 0.01.
Compared with control, ‡p < 0.05, ¦p < 0.01.
Table III. The neurocognitive test scores at baseline and after sixth month of supplementation with vitamin B12 or control.
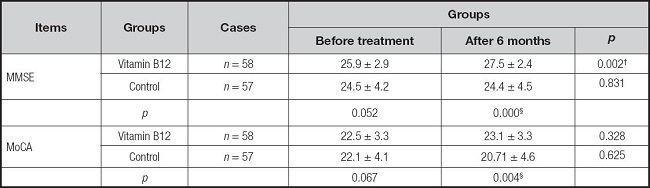
MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment.
Variables are presented as mean ± SD.
Compared with before treatment, *p < 0.05, †p < 0.01.
Compared with control, ‡p < 0.05, ¦p < 0.01.
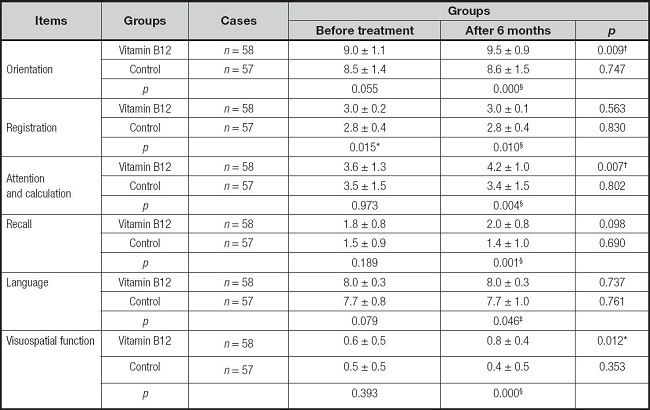
The changes in the six domains of MMSE scores between the two groups are compared in table IV. At six months, the B12 treatment group showed a significantly improvement in orientation, attention, and calculation compared to before treatment (p < 0.01). In addition, visuospatial function improved significantly in the B12 treatment group compared to that before treatment (p < 0.05). Specifically, at month 6, B12 treated group improved significantly orientation, registration, attention and calculation, recall, visuospatial function (p < 0.01), and language (p < 0.05) compared to the control group.
DISCUSSION
Our results showed that vitamin B12 supplementation improved cognitive function in middle-aged and elderly individuals over a six-month period, especially in attention and calculation (p < 0.01) and visuospatial function (p < 0.05). In addition, vitamin B12 supplementation prevents the progression of cognitive impairment in patients. All of these results support vitamin B12 as a part of routine assessments for treatment candidates of cognitive impairment, which can be easily and rapidly determined at outpatient departments. For bedside practicality, we also evaluated the seven domains of MoCA-30 in each group respectively. If confirmed in larger and longer-term randomized trials, it is expected to be widely used in clinical practice.
The relationship between vitamin B12 and cognitive impairment or dementia is likely multifactorial. The mechanisms may include methylation disorders, demyelination, neurotransmitter and neurotrophic factor synthesis disorders, accumulation of toxic metabolites such as tHcy and methylmalonic acid, and immune system dysfunction (15,17,29-31).
Cognitive decline in older adults is a public health concern. To date, several studies have evaluated the effect of B vitamins as a treatment for cognitive impairment or dementia, but only the VITACOG and FACIT trials have reported the benefits of treatment (32,33). Recently, a Korean study found a clear association between low vitamin B12 levels and progressive cognitive impairment in 202 patients with cognitive decline, with an increased level of vitamin B12, which could slow the progression of cognitive decline to dementia (31). Moreover, 56 % of 202 patients had a level between 100 and 200 pg/ml, and 15.3 % had a vitamin B12 level between 50 and 100 pg/ml (31). In addition, 7.5 % of dementia patients had a vitamin B12 deficiency (vitamin B12 levels < 200 pg/dl). In our study, we found that 29.6 % of patients with cognitive impairment had vitamin B12 deficiency (vitamin B12 levels ≤ 180 pg/ml). However, it must be noted that this ratio may be much lower than the true ratio because the standard of vitamin B12 levels used in this study is 180-900 pg/ml, which may lead to some patients with metabolic vitamin B12 deficiency being ignored by us. In addition, in our study, 50.4 % of patients with cognitive impairment had metabolic vitamin B12 deficiency (vitamin B12 levels ranged from 180 to 400 pg/ml). In 2016, early treatment of metabolic vitamin B12 deficiency was suggested as an important strategy to prevent dementia (15). Thus, future studies should focus on the association between vitamin B12 levels and cognitive dysfunction.
In our study, we found that vitamin B12 supplementation improved MMSE scores in middle-aged and elderly individuals with cognitive decline, over a six-month period, especially in attention and calculation and visual-constructional ability. This suggests that vitamin B12 supplementation could improve the function of the frontal lobes of the brain, which is consistent with the results of other studies (35,36). Furthermore, studies have reported that vitamin B12 deficiency could manifest with the symptoms of frontotemporal dementia and that they are completely reversible after substitution therapy (36,37), further supporting our results. Therefore, the effects of treatment have shown that early identification and alternative treatment can significantly reverse symptoms, which is an important step toward a healthy mental state.
The strength of our study is that it explicitly controls for key confounders, such as using any nutritional supplementation, the diseases which influence cognitive score; the scores of different aspects of cognitive function were compared in detail, and the results that support our idea of improved cognition with replacement therapy. Our study had several limitations. First, as a sample of a relatively small number of patients with cognitive decline who underwent vitamin B12 testing for various clinical indications, it lacked a systematic collection of vitamin B12 data, potentially leading to selection bias. Second, our experiment was conducted in the outpatient department of an affiliated university hospital. Most participants in the study had a higher level of education, which might have led to selection bias. In addition, due to the influence of COVID-19, the number of outpatient clinics has decreased, resulting in a small number of patients being included and lost visits, which cannot reflect the real results in our region. Finally, the follow-up period was only six months, and a longer follow-up period was needed to assess the improvement in cognitive function.
CONCLUSION
In summary, in our study it was noted that with vitamin B12 supplementation, there was improvement in cognitive function scores in middle-aged and elderly individuals with cognitive decline although no definite conclusion can be made as the follow-up period was very short. Moreover, vitamin B12 treatment may improve frontal function in patients with cognitive decline. Vitamin B12 levels should be investigated in all patients with cognitive impairment. Larger and longer-term randomized trials on vitamin B12 are needed.