Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.3 Madrid mar. 2004
| RECOMENDATIONS OF CLINICAL PRACTICE |
Endosonographic characteristics of submucosal tumors (SMT).
Approach and algorithm
M. J. Varas Lorenzo
Unit of Eco-Doppler and Ecoendoscopy. Centro Médico Teknon.
Unit of Ecoendoscopy. Centro Internacional de Medicina Avanzada (CIMA). Barcelona, Spain
AED Action Protocol for Gastrointestinal Ultrasonography. Coordinators: M. Gómez Rubio, MD and José Souto Ruzo, MD.
Varas Lorenzo MJ. Endosonographic characteristics of submucosal tumors (SMT). Approach and algorithm. Rev Esp Enferm Dig 2004; 96: 215-218.
Recibido: 29-12-03.
Aceptado: 12-01-04.
Correspondencia: M. J. Varas Lorenzo. Unidad de Eco-Doppler y Ecoendoscopia. Centro Médico Teknon. Unidad de Ecoendoscopia. Centro Internacional de Medicina Avanzada (CIMA). Barcelona
INTRODUCTION
Submucosal lesions (SMLs) of the upper gastrointestinal tract (1-4) represent a significant diagnostic and therapeutic challenge to both endoscopists and gastroenterologists. Such lesions may be assessed by using multifrequency transendoscopic miniprobes, or endoscopic ultrasonography (EUS), either radial or linear.
Fine-needle puncture-aspiration (FNA) of SMTs should be guided by only linear EUS.
EUS YIELD
Submucosal lesions or abnormalities:
1. Can EUS or endoscopic ultrasonography recognize submucosal lesions and differentiate between extrinsic compression (spleen, liver, etc.) and gastrointestinal submucosal tumors?
Yes, it can with a sensitivity greater than 90% (3,5). According to our group's cumulative experience (more than 140 cases), sensitivity was 94%.
2. Can EUS recognize a lesion's exact origin, size, and ultrasonographic pattern?
Yes, EUS can establish whether a tumor is within the gastrointestinal wall or otherwise. Regarding submucosal tumors (lipomas, myoid tumors), EUS can identify the layer a tumor originates in as well as its size, and is also a good technique for the investigation of gastroesophageal SMT endosonographic pattern (score of 9 at Monaco Consensus in 2000) (4). It also successfully differentiates liquid from solid lesions.
3. Can EUS differentiate between benign and malignant (stromal) SMTs?
It can be done with a sensitivity above 80% (6-8). EUS was assigned a score of only 6 at the Consensus Meeting held in 2000 (4).Classic malignant characteristics include: size greater than 3 cm, nodular shape, irregular borders, surface ulceration, heterogeneous pattern, and presence of anechoic foci indicating intratumor necrosis (6). According to the experience collected by us and other authors (7), benign SMTs are usually smaller than 4 cm in size. The likelihood of malignancy is 0-11% when none of the following signs are present: size greater than 4 cm, irregular borders, echogenic foci greater than 3 mm in size, and cystic foci greater than 4 mm in size. Should more than two of these signs be present, the likelihood of malignancy rises to 80-100% (7). The finding of irregular borders, cystic shapes, or presence of adenopathies indicates malignancy with a positive predictive value (PPV) of 100% (8).
4. Which is the role of transendoscopic miniprobes (MPs) in assessing SMTs?
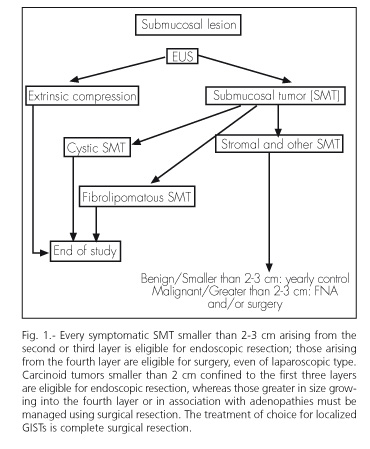
Two independent studies demonstrated that accord between EUS and MPs was very high. In our experience, the sensitivity of EUS and MP was 95%, and accord was almost 100% (10). Therefore, we feel that MPs may be safely used in daily clinical practice to assess SMTs, as most of these lesions are usually benign (9), small in size (less than 2 cm), and not in need of any treatment. 5. How are SMTs approached and managed? An algorithm? (Fig. 1).
Once an imaging technique (radiography, ultrasonography, computerized tomography, magnetic resonance imaging, endoscopy) has detected the presence of a submucosal lesion, EUS must be unavoidably used to definitely confirm the presence of an SMT, attempt to characterize the lesion's histology, and orient regarding its bening or malignant nature. Studies will be terminated if the finding is a compression or a cystic or lipomatous SMT.
Otherwise, surveillance with yearly follow-up or treatment by either endoscopic or surgical means is recommended, following a previous FNA with immunohistochemical testing [CD34, c-kit (CD117, Ki-67)] (11,12). Biopsy and FNA are of limited value, particularly for gastrointestinal stromal tumors (GISTs). A classification to predict aggression based on size, site, and mytotic index has been proposed for these tumors (13).
Symptomatic SMTs (bleeding, obstruction, etc.) smaller than 2-3 cm in size and originating in the second or third layer of the gut are eligible for endoscopic resection (14,15) with low risk. Approximately, 75% of resected hypoechogenic SMTs are in fact GISTs, and at least 5% of them are malignant (9).
CONCLUSIONEUS is essential in the assessment of SMLs. Most SMTs are benign and need not be treated. FNA may be used for cases not properly characterized by ultrasonography or doubtful. It is of limited value for GISTs. EUS-provided information may help decide on either endoscopic or surgical treatment.
REFERENCES
1. Yasuda K, Nakajima M, Yhosida S, Kiyota K, Kawai K. The diagnosis of submucosal tumors of the stomach by endoscopic ultrasonography. Gastrointest Endosc 1989; 35: 10-5. [ Links ]
2. Kawamoto K, Ueyama T, Iwashita I, et al. Colonic submucosal tumors: Comparison of Endoscopic US and target air-enema CT with barium enema study and colonoscopy. Radiology 1994; 192: 697-702. [ Links ]
3. Rösch T. Endosonografía en los tumores de la submucosa de vías gastrointestinales superiores: revisión de publicaciones. Clin Endoscopia Norteamérica 1995; 3: 593-7. [ Links ]
4. Lambert R, et al. EUS 2000. International Workshop on the clinical impact of endoscopic ultrasound in gastroenterology. Endoscopy 2000; 32: 549-84. [ Links ]
5. Varas MJ, Maluenda MD, Pou JM, Abad R, Turró J, Espinós JC. Valor de la ultrasonografía endoscópica en el estudio de los tumores submucosos del tracto digestivo. Gastroenterol y Hepatol 1998; 21: 121-4. [ Links ]
6. Yamada Y, Kida M, Sakaguchi T, et al. A study on myogenic tumor of upper gastrointestinal tract by endoscopic ultrasonography. Dig Endosc 1992; 4: 396-408. [ Links ]
7. Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc 1997; 45: 468-73. [ Links ]
8. Palazzo L, Landi B, Séller C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumors. Gut 2000; 46: 88-92. [ Links ]
9. Nickl N, Gress F, McClave S, et al. Hypoechoic intramural tumor study: final report. Gastrointest Endosc 2002; 55: AB98. [ Links ]
10. Varas MJ, Abad R, Maluenda MD, Pou JM. Ultrasonografía Endoscópica versus Minisonda en el estudio de los tumores submucosos del tracto digestivo. Rev Esp Eco Dig 2000; 2: 119-24. [ Links ]
11. Matsui M, Goto H, Niwa Y, et al. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumours. Endoscopy 1998; 30: 750-5. [ Links ]
12. Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemiocal análisis. Gastrointest Endosc 2002; 55: 37-43. [ Links ]
13. Miettine M, El-Rifai W, Sobin LH, Lasota J. Evaluation og malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2003; 33: 478-82. [ Links ]
14. Hyun JH, Jeen YT, Chun HJ, et al. Endoscopic resection of submucosal tumor of the esophagus: results in 62 patients. Endoscopy 1997; 29: 165-70. [ Links ]
15. Hunt G, Smith P, Faigel D. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2002; 56: S102. [ Links ]











 texto en
texto en