Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.104 no.3 Madrid mar. 2012
https://dx.doi.org/10.4321/S1130-01082012000300015
LETTERS TO THE EDITOR
Tuberculous monoarthritis after treatment with adalimumab in Crohn's disease
Monoartritis tuberculosa tras tratamiento con adalimumab en enfermedad de Crohn
Key words: Tuberculosis monoarthrosis. Adalimumab. Crohn.
Palabras clave: Monoartritis tuberculosa. Adalimumab. Crohn.
Dear Editor,
Anti-tumor necrosis factor alpha (TNF-α) drugs have demonstrated their usefulness in treating some autoimmune rheumatic diseases and inflammatory bowel disease. Its affinity to TNF-α prevents the activation of proinflammatory cascade. Adalimumab is a human monoclonal antibody that is administered subcutaneously. Several safety studies have concluded that it is well tolerated but not without adverse effects. Most of them are mild although there are cases of lymphoma, demyelinating processes and infections. Active tuberculosis is a contraindication to its administration according to the technical data sheet. However, latent tuberculosis requires initiating prophylaxis against tuberculosis. Below we present a case of tuberculous monoarthritis of the wrist, in the context of miliary tuberculosis, in a patient with Crohn's disease treated with adalimumab.
Case report
This is a 55 year old patient diagnosed with ileocolic Crohn's disease thirteen years ago that required resection of terminal ileum and cecum at the time of diagnosis.
During this period, he had a good control of his disease with azathioprine 100 mg daily. Despite being treated with azathioprine, he suffered an episode of diarrhea and diffuse abdominal pain; therefore, so we decided to associate an anti-TNF.
After verity that serology for HBV, HCV, Mantoux and booster were negative, and that chest radiograph was normal, we introduced adalimumab 80 mg the first day and then 40 mg every two weeks.
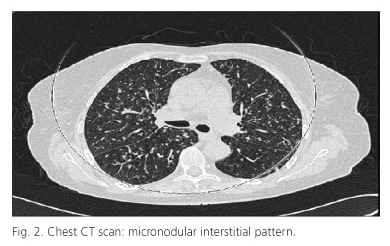
Two months after initiation of adalimumab, the patient was admitted to the rheumatology department of our hospital with fever, functional disability and local pain in left wrist that had not subsided with anti-inflammatory drugs. The analytical parameters were normal except for an ESR of 54 mm/h. The resonance of the wrist showed a moderate synovitis with involvement of the flexor tendon sheaths with no signs of osteomielitis. Blood culture and urine tests were negative. The chest radiograph showed a micronodular pattern in both lungs (Fig. 1) and chest CT (Fig. 2) showed a disseminated micronodular interstitial pattern with mediastinal lymph nodes (the largest in diameter). For an episode of syncope he underwent brain MRI that showed multiple microabscesses in cerebellar region and the brain stem. The new Mantoux was positive and a culture of bronchoalveolar lavage showed the presence of Mycobacterium tuberculosis. After debridement of the left carpus and the initiation of tuberculostatic therapy, the patient had a good outcome.
Discussion
The appearance of miliary tuberculosis a few weeks after initiation of the treatment with adalimumab leads us to believe that the patient already had a latent tuberculosis that was not detected in the study before biological therapy. The reactivation of infection is presented in an unusual way, as monoarthritis.
Patients in whom immunosuppressive therapy is initiated should be monitored for possible infections before, during and even after stopping treatment.
Alejandro Martínez-Caselles, Cristina Martínez-Pascual, María Muñoz-Tornero,
Antonio Sánchez-Torres and Fernando Carballo-Álvarez
Department of Gastroenterology. Hospital Universitario Virgen de la Arrixaca. Murcia, Spain
References
1. Blanco Pérez JJ, Aranda Torres A, Pego Reigosa JM, Núñez Delgado M, Temes Montes E, Guerra Vales JL. Pulmonary tuberculosis associated to adalimumab: a study of 3 cases. Arch Bronconeumol 2010;46:203-5. [ Links ]
2. Ferran M, Pujol RM. Seguridad del adalimumab. Actas Dermosifiliogr 2008;99(Supl.3):15-24. [ Links ]
3. Colombel JF, Sandborn WJ, Panaccione R, Robinson AM, Lau W, Li J, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflamm Bowel Dis 2009;15:1308-19. [ Links ]











 texto en
texto en