Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.106 no.1 Madrid ene. 2014
https://dx.doi.org/10.4321/S1130-01082014000100002
Evaluation of the adequacy and diagnostic accuracy of the histology samples obtained with a newly designed 19-gauge EUS histology needle
Evaluación de la adecuación y seguridad diagnóstica de las muestras histológicas obtenidas con una aguja recientemente diseñada de 19G guiada por ecoendoscopia
Julio Iglesias-García1,2, Ihab Abdulkader3, Jose Lariño-Noia1,2 and J. Enrique Domínguez-Muñoz1,2
1Gastroenterology Department
2Foundation for Research in Digestive Diseases (FIENAD)
3Pathology Department. Hospital Universitario de Santiago de Compostela. A Coruña, Spain
Additional remark: For this trial Cook Medical supported with products.
ABSTRACT
Background: Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) is an accurate technique for sampling intraintestinal and extraintestinal lesions. However, cytology possesses certain limitations, which may be overcome if histological specimens are provided to the pathologist.
Aim: The aim of the study was to evaluate the accuracy of a newly developed 19G histology needle.
Methods: Retrospective analysis of a prospectively collected data base including patients who underwent EUS-guided biopsy with the 19G ProCoreTM histology needle for the evaluation of intraintestinal or extraintestinal lesions. Samples were obtained after one needle pass, recovered into ThinPrep® and processed for histological analysis. Results were compared to the gold standard of surgical histopathology, or global pathological, clinical and radiological assessment, and follow-up in non-operated cases. Results are shown as mean ± SD. Percentage of optimal samples for histological evaluation and the overall diagnostic accuracy were evaluated.
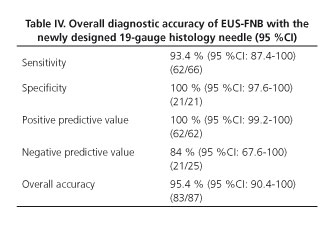
Results: 87 patients (mean age 62.9 years, range 25-88 years, 36 woman) were included. Lesions mean size was 41.6 ± 21.3 mm. 66 lesions (75.9 %) were considered as malignant and 21 (24.1 %) as benign. EUS-guided biopsy was feasible in all cases (100 %). Sample quality was adequate for histological assessment in 82 lesions (94.2 %). In the remaining cases the sample was adequate for cell-block evaluation. Sensitivity, specificity, PPV, NPV, and overall accuracy for malignancy were 93.4 %, 100 %, 100 %, 84 %, and 95.4 %, respectively. There were no complications related to the procedure.
Conclusion: The EUS-guided biopsy with the 19G histology needle provides with an optimal core sample for histological evaluation allowing a high histopathologic diagnostic accuracy.
Key words: Endoscopic ultrasound. Fine needle biopsy. Histology.
Introduction
EUS is a sensitive method for detecting intraintestinal and extraintestinal masses and peri-intestinal lymph nodes (1-4). EUS-guided fine needle aspiration (EUS-FNA) has a diagnostic accuracy ranging from 60 % to 90 % (5-7). Cytological evaluation of the samples obtained by FNA allows evaluating cellular findings suggestive of malignancy, like anisonucleosis, nuclear membrane irregularity and nuclear enlargement. However, inflammation causes a reactive and regenerative process leading to cellular changes undistinguishable from well-differentiated neoplasia. Moreover, certain neoplasms such as lymphoma and stromal tumors require histological samples for diagnosis, since tissue architecture and cell morphology are essential in these cases for accurate pathological assessment and immunohistochemical analysis (5,8-10).
Whereas FNA only provides cells largely disrupted from their original arrangement, larger-caliber cutting needles allow for core biopsy specimens (11-18). These specimens have been obtained by several routes (percutaneous, intraluminal and surgical) (16-22), and safety and accuracy of cutting biopsy have been previously demonstrated (16,22-24).
Various EUS-guided techniques have been explored to retrieve tissue specimens, including FNA and Tru-Cut needles, with variable success and complication rates (25-32). Of particular interest is the Quick-Core® needle, designed to operate through an echoendoscope. EUS-guided use of Quick-Core® needle has allowed the safe obtaining of histological samples representative of the target organs (33,34). However, there are certain drawbacks with this needle restricting its use in clinical practice. Most importantly, its diagnostic yield is strongly limited for lesions located in the head of the pancreas due to mechanical friction of the needle firing mechanism ensuing from the bended scope position (35-38). Nowadays a new 19-gauge fine needle biopsy needle (ProCoreTM, Cook Endoscopy Inc., Limerick, Ireland) device has been designed. Feasibility, yield and high diagnostic accuracy of this needle have been recently report in a large series of consecutive patients in a multicenter study, but different methodologies were used in each center (39). Aim of the present study was to evaluate the diagnostic accuracy of this newly developed 19-gauge histology needle in a single center using a homogeneous methodology. Targets included intestinal and extraintestinal mass lesions and peri-intestinal lymph nodes.
Material and methods
Design
A retrospective analysis of a prospectively collected database of the diagnostic accuracy of the EUS-guided fine needle biopsy with the newly designed 19-gauge histology needle for the evaluation of intraintestinal or extraintestinal mass lesions and/or peri-intestinal lymph nodes was designed.
Subjects
All patients submitted over a time period of 18 months to the EUS Unit of the Department of Gastroenterology of the Hospital Universitario de Santiago de Compostela, Spain for the evaluation of solid, and in which the new 19-gauge histology needle was used, were included in the study. Solid lesions bigger than one centimeter in diameter were finally included. Lesion smaller than 1 cm, cystic lesions and masses with a predominant cystic component, as well as lesions biopsied with on-site cytopathological evaluation were excluded from the study.
Methods
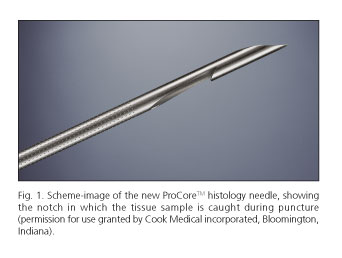
EUS-guided fine needle biopsy (FNB) was performed using a convex array echoendoscope (Pentax EG-3870UTK®) by two well-trained endosonographers (J.I.G. and J.L.N). Tissue acquisition was done with the newly designed 19-gauge Echotip® ProCore High Definition Biopsy Needle, featuring ProCore reverse bevel technology (Fig. 1). The needle is 1.705 m long, made of stainless steel with a nitinol stylet. The stylet running through the cannula of the needle is matching the tip bevels. The sheath is 5.2 Fr and the reverse bevel length is 4 mm.
Tissue acquisition was done according to a well-defined and homogeneous protocol. According to it, and after the target lesion was endosonographically visualized and the region scanned for vessels using color and pulsed Doppler, biopsy was performed either from esophagus, stomach, duodenum or rectum depending on the location of the lesion. The newly designed histology needle was advanced into the target tissue under endosonographic guidance with the stylet fully inside the needle. Once the lesion was penetrated, the stylet was removed and suction was applied for 10 to 20 seconds using a 10 mL syringe while moving the needle to and fro within the lesion 3-4 times. Suction was released before removing the needle. One single needle pass was performed. Tissue samples were recovered in a liquid-based preparation, ThinPrep® (Hologic Corp., Bedford, MA) by flushing the needle with 5 cc of saline. All samples were processed at the Pathology Department of our centre for histological analysis. There was no pathologist present in the endoscopy room and biopsy samples were recovered and stored for further processing by the endoscopist. Biopsy samples were evaluated by the same pathologist with particular interest and expertise in evaluating tissue materials obtained via EUS. Samples were embedded in paraffin. Tissue sections of 3-4 µm were stained by the hematoxylin-eosin technique for morphological evaluation and/or different immunohistochemical analysis (cytokeratine, CD56, chromogranin and synaptophisyn for endocrine tumors; CD20, CD3, bcl2, for lymphoma, or cytokeratin and TTF1 for non-small cell lung cancer). If a core for histological evaluation was not obtained, the same material was processed as cellblock for cytological evaluation.
Gold standard reference method
A final diagnosis was defined according to the following reference methods: a) Histology of surgical specimens in cases who underwent surgery; b) a definitely positive pathological finding for malignancy at EUS-guided biopsy sample together with compatible EUS and computed tomography (CT) scan findings for malignant disease in unresectable tumors; and c) EUS and CT scan findings at entry, clinical presentation, and a minimum follow-up period of 6 months including EUS-guided biopsy and CT scan, for final diagnosis of benign disease in cases of benign pathology at initial EUS-guided FNB.
The study was conducted in accordance with the Declaration of Helsinki and its amendments, and Good Clinical Practice guidelines. All patients provided written informed consent to the study. EUS and EUS-FNA for the evaluation of intraintestinal or extraintestinal mass lesions and/or peri-intestinal lymph nodes are routine procedures in clinical practice. The use of the new histology needle instead of the standard cytology needles adds no risk or inconvenience to the patient (39).
Variables evaluated
Primary outcome was the percentage of cases in which a correct final histological diagnosis was obtained.
The quality of the sample was evaluated as a secondary outcome. The pathologist classified the quality of the samples as follows: Grade 1 - no sample obtained; grade 2 -poor sample, not suitable for histological evaluation, but enough for cytological analysis; grade 3 - good sample, suitable for histological evaluation, but providing an incomplete tissue architecture of the target lesion (i.e. without a real core or when the core was fragmented and difficult to be evaluated); grade 4 - excellent sample, suitable for histological evaluation, providing tissue architecture of the target lesion (i.e. with a real core) (38,39). The percentage of cases in which the pathologist was able to perform an immunohistochemical analysis was also evaluated as secondary outcome.
Visibility of the needle during the puncture, ease of FNB needle insertion through the scope, ease of FNB needle removal from the scope, ease of removal the stylet after advancement of the FNB needle in the target lesion, and optical impression of the tissue sample obtained after puncture by the endoscopist were also evaluated.
Patients were monitored at the endoscopy unit for two hours after the procedure for the evaluation of complications. Further follow-up was performed by evaluating the electronic clinical record of the patients at days 3, 7 and 15 after the EUS-guided FNB. Electronic clinical record includes any clinical episode of every patient being attended at any hospital of the regional health system, and therefore is a highly reliable tool for checking for any clinical event both related and unrelated with the procedure.
Data analysis
Results are shown as percentage and 95 % confidence interval (CI). Normally distributed variables are presented as mean with standard deviation and range. A descriptive analysis is performed. Sensitivity, specificity, positive predictive value, negative predictive value, and overall accuracy were calculated. Statistical analyses were performed with the software SPSS 20.0 (Chicago, Illinois).
RESULTS
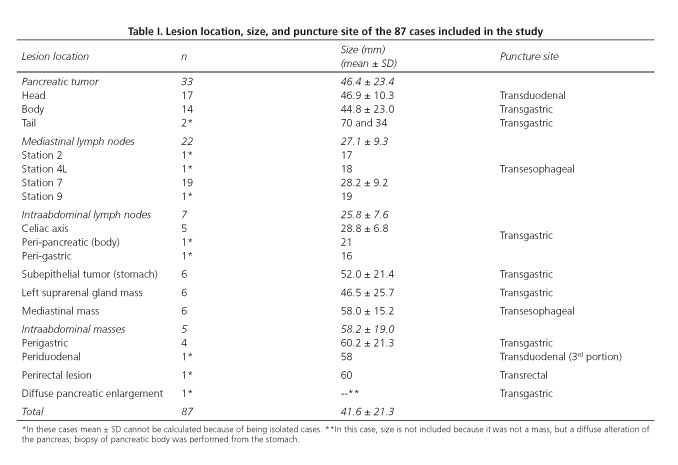
Of the 494 EUS-guided biopsies performed over the study period, 87 procedures were performed using the 19-gauge histology needle in 87 patients (mean age 62.9 years, range 25-88 years, 36 female and 51 male). Mean size of the 87 lesions evaluated was 41.6 ± 21.3 mm. Seven lesions (8.0 %) were smaller than 2 cm. Location and size of the lesions, as well as puncture site are described in table I. Sixty-six lesions (75.9 %) were finally considered as malignant and 21 lesions (24.1 %) as benign. Thirty-one of these patients were previously reported as part of a multicenter trial (38).
Final diagnosis was based on surgical specimens in 24 cases (18 malignant and 6 benign lesions). In 48 cases final diagnosis of unresectable malignant tumors was based on the cytological and/or histological findings after EUS-biopsy with definite proof of malignancy, together with compatible EUS and CT scan findings. Finally, in 15 cases without proof of malignancy at initial EUS-guided biopsy, final diagnosis was based on compatible EUS and CT scan findings for benign disease, clinical presentation, and a follow-up time of 6.6 months (range 6-8), including EUS-FNA and CT scan at the end of follow-up.
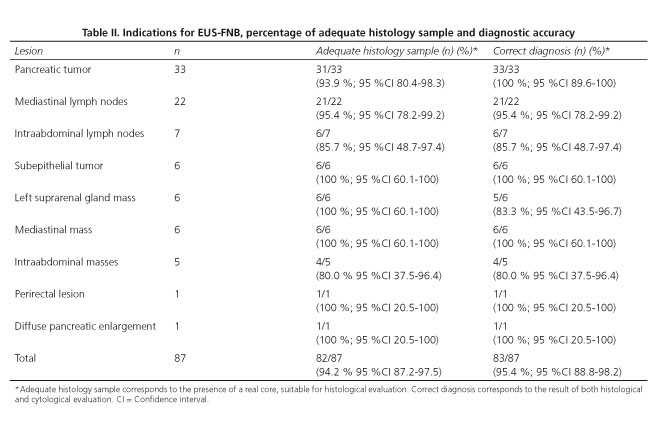
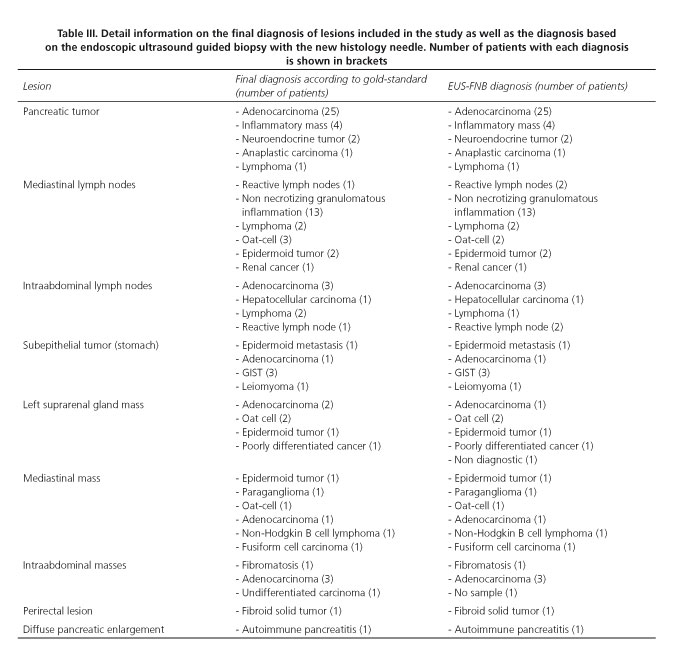
A final diagnosis was provided in all 87 cases (100 %), and in 83 of them (95.4 %; 95 %CI 88.8-98.2 %) this diagnosis proved to be correct according to the gold standard. Table II shows the percentage of correct diagnosis. According to lesion size, EUS-FNB allowed diagnosing accurately 6 out of 7 tumors smaller than 2 cm (85.7 %; 95 %CI 48.7-97.4), and 77 out of 80 tumors ≥ 2 cm in size (96.2 %; 95 %CI 89.5-98.7) (p = 0.283). Detailed information regarding the diagnosis based on the gold standard and on the EUS-FNB is provided in table III. Diagnostic accuracy of the FNB with the evaluated needle for the detection of malignancy is shown in table IV.
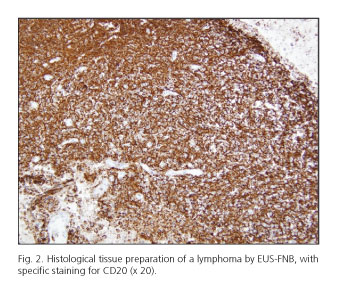
When evaluating the sample optically after the needle content was flushed into ThinPrep®, a tissue core could be observed in 76 cases (87.3 %); a tissue core mixed with blood in 10 cases (11.5 %), and scarce sample in 1 case (1.2 %). A sample suitable for pathological evaluation was obtained from 86 lesions (98.8 %). Sample quality according to the pathologist was adequate for full histological assessment in 82 lesions (94.2 %) (Table II and figure 2). In the remaining cases the sample was adequate for cytological evaluation (processed as cell-block). The pathologist classified the quality of the samples as follows: Grade 1 in one case (1.2 %); grade 2 in 4 cases (4.6 %); grade 3 in 15 cases (17.2 %); and grade 4 in 67 cases (77 %).
EUS-guided biopsy was technically feasible in all 87 cases (100 %) and through all different locations (esophagus, stomach, duodenum and rectum). There were no complications related to the technique.
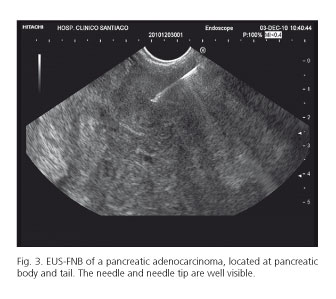
The biopsy device was easy to insert into the scope in all 87 cases (100 %). The needle emerged from the scope easily in 75 cases (86.2 %), with difficulty in 11 cases (12.6 %), and with great difficulty in one case (1.2 %). This appeared to be only a problem when punctures were performed through the duodenum (60 %) with the scope in a bended position, compared to other locations (60 % vs. 4.2 %, p < 0.001). Removing the stylet after puncturing the lesion was easy in 73 cases (83.9 %), hard in 12 cases (13.8 %), and very hard in 2 cases (2.3 %). Difficulties in removing the stylet was mainly related to punctures performed through the duodenum, compared to other locations (80 % vs. 2.8 %, p < 0.001). The visibility of the needle was judged as optimal in all 87 cases (100 %) (Fig. 3).
Discussion
The present study shows that an adequate tissue sample for full histological evaluation can be obtained in almost every patient from a variety of lesions with the use of a newly designed EUS 19G histology needle. The overall diagnostic accuracy of the pathological evaluation for the detection of malignancy of the tissue obtained by this needle was of 95.4 %.
EUS-guided tissue sampling has emerged as a valuable technique for many indications (4). Conventional EUS-FNA has certain limitations. Sensitivity significantly decreases by 10-15 % without an on-site pathologist to evaluate the sample obtained during the procedure (40-42). Without on-site evaluation, the recommended number of passes is 5-7 for solid pancreatic lesions and 2-3 for lymph nodes (34,40,41). However, the on-site pathologist allows reducing the number of needle passes and increasing the diagnostic accuracy of the EUS-guided FNA (42). For pancreatic tumors, additional needles and punctures are needed in 15 % of cases, increasing the overall procedural time (9). The lack of cellular arrangement and preserved tissue architecture in cytology samples limits the possibility of making an adequate diagnosis in a relevant proportion of cases (37). In fact, the yield of cytology in certain tumors such as lymphoma, poorly differentiated adenocarcinoma, and stromal tumors is limited. In this setting, immunohistochemistry is required for final diagnosis and tumor subtyping. Sensitivity of EUS-FNA is also uniformly poor when performed in certain anatomic locations such as thickened gastrointestinal wall or focal intramural lesions (5,43).
In order to avoid these problems related to cytological evaluation, several attempts have been undertaken to obtain EUS-guided core tissue specimens for histopathological analysis. Initial attempts were associated with the use of both large caliber (18-21-gauge) needles (25,26) and the standard 22-gauge needle (28). Binmoeller et al. (26) were able to obtain adequate tissue core specimens in 40 out of 45 patients with pancreatic masses by using an 18-gauge needle, but sensitivity for detection of malignancy was only 53 %. On the other hand, we were able to obtain an adequate tissue sample for histological diagnosis of pancreatic masses in 95 % of patients after EUS-FNA by recovering the pancreatic EUS-FNA specimen by injecting saline through the needle (28). Three studies have been able to obtain adequate histological samples by using the 19-gauge standard cytology needle. Yasuda et al. (29) could obtain histological samples with the standard needle in almost every evaluated patient, with an overall diagnostic accuracy of 98 %, but by doing more than one pass. In addition, only FNA from lymph nodes were included in that study, what cannot be directly compared to pancreatic lesions (29). In our series, we have not only included lymph nodes from various locations (obtaining a similar accuracy with less passes), but also 33 pancreatic lesions. Larghi et al. (30) developed a technique for tissue acquisition by removing the stylet before needle insertion into the EUS working channel to increase flexibility. With this method adequate samples were obtained in 97.5 % of the cases (similar to the present study), being successful in 98.9 %, and with an overall accuracy over 90 %. However, authors needed a mean of 2.8 passes. Even more, the technique described can be associated with more complications since removal of the stylet can be associated with a bending of the needle during the puncture. Finally, Stavropoulos et al. (31) were able to perform a EUS-guided liver biopsy by using a 19-gauge needle in 22 patients. They obtained adequate liver cores in 91 % of patients, with a median specimen length of 36.9 mm. Authors needed a median of 2 passes to obtain these results, whereas in our experience, only one pass was needed.
Up to now, the most widely used needle for obtaining histological samples has been the Quick-Core® needle (32-34,38). Although biopsy with Quick-Core® needle has no clear advantage over EUS-FNA in terms of overall sensitivity and diagnostic accuracy, it does provide with a more specific diagnosis in selected cases with less needle passes (29,37,44). A combination of EUS-biopsy with Quick-Core® needle and EUS-FNA has shown a higher overall diagnostic yield than any of the two needles alone (45-47). The overall accuracy of EUS-biopsy in these later studies ranges from 61 % to 84 %. Contrarily to the Quick-Core® needle, the presence of the reverse bevel technology in the new histology needle evaluated in the present study allows obtaining a core by cutting the tissue in each to and fro movements during one single needle pass.
First results with this new histology needle regarding feasibility, yield and diagnostic accuracy have been described in a multicenter study (38). Published data are comparable, or even better, to those described with the previous needles, both cytology and Quick-Core®. Adequate sample for full histological evaluation was obtained in 89.5 % of the evaluated lesions, and the overall diagnostic accuracy was 85.9 %. In our study, we have been able to obtain an adequate sample for histological evaluation in all lesions but one, with an excellent sample, providing real tissue architecture in 77 % of cases. This was associated with a very high diagnostic accuracy (95.4 %), improving the previously reported diagnostic yield of standard cytology (5-7) and other biopsy needles (44-46). As a relevant issue, these data were obtained after performing only one pass into the target lesion.
Although in the present study we have used 19G histology needles, thinner needles (22G and 25G), which are also commercially available, may be especially useful for lesions with a difficult access (e.g. from the second portion of the duodenum) and with the echoendoscope in a bended position.
Another important issue is that this high diagnostic accuracy was obtained from all sorts of lesions. Similar to the previous multicenter study (39) we could correctly diagnose and sub-classify different types of lymphomas, subepithelial tumors, benign lymph nodes (sarcoidosis vs. tuberculosis) and pancreatic solid tumors (metastasis, pancreatic adenocarcinoma, and/or autoimmune pancreatitis). This illustrates the potential benefit and impact on patient management of this novel histology needle.
An additional advantage of this device is that overcomes certain limitations described with the use of the Quick-Core® needle. Due to the rigidity of the needle that limits the degree of the echoendoscope tip deflection required to bring the target lesion into an adequate position for puncture, the diagnostic yield of the Quick-Core® needle is strongly limited for lesions that should be punctured from the duodenum (32,35,37,38). Also, the bended scope position induces considerable friction within the needle firing mechanism that may impair its proper function. With the newly designed Pro-CoreTM needle, puncturing from a duodenal position appears to be easier. In the previous multicenter study (38), puncturing from the duodenum was feasible in 94.3 % of the cases and in our series this was possible in all cases. However, puncturing from a duodenal position still remains more difficult and in some cases the needle should be pushed out of the scope into the stomach before advancing the scope into the duodenum. EUS-FNB through the esophagus, stomach and rectum was easy and uneventful in all cases. A critical point in the present study is the use of a well-defined gold-standard reference method. Ideally, histology from surgical specimen should be used as gold standard. However, this cannot be obtained for ethical reasons in patients in whom surgery is not indicated. In these specific cases, either a positive FNB result for malignancy together with EUS and CT findings of unresectable tumor, or a clinical follow-up of at least 6 months with repeated imaging procedures (EUS and CT) and EUS-FNA for benign lesions were used. The inclusion of the EUS-guided biopsy as one of the diagnostic criterion of the reference method is a weakness of the present study. In this way, accuracy data can be overestimated. Nevertheless, the information provided by the clinical evaluation and imaging procedures at inclusion and after follow-up in non-operated cases allowed us to properly classify these lesions, thus minimizing the potential bias. It is also important to point out that this is a single center study, and that both endosonographers and the pathologist are experts in EUS-guided FNA and in the evaluation of samples obtained by this procedure. This may clearly play a role in the high diagnostic accuracy obtained. Diagnostic accuracy of EUS-FNB with this 19G histology needle was not significantly influenced by the lesion size, although the number of lesions smaller than 2 cm included in the study does not allow us to draw definite conclusions. Safety of EUS-FNA is well established (48) and complication rates range from 1 % to 2.5 % (49). Small series have reported complication rates of EUS-guided Quick-Core® biopsy ranging from 2 % to 4 %, with the largest study showing a complication rate of 2.4 % (38). One concern with this newly designed needle is the risk of contamination of the surrounding tissues during puncture. This is especially true when puncturing lesions smaller that 1 cm, since the needle hole is located between 5 mm and 9 mm from the tip of the needle. In the present study the smallest lesions included were 12 mm in size. In our present series of 87 cases there were no complications associated to the procedure. Therefore, the use of EUS-FNB needle seems as safe as the standard FNA or Quick-Core® needle. In conclusion, performing a EUS-guided biopsy with the newly designed Pro-CoreTM needle is safe and accurate for histopathology diagnosis of intraintestinal and extraintestinal mass lesions. It offers the possibility to obtain a core sample for histological evaluation in the majority of cases, with an overall diagnostic accuracy of over 95 %. References 1. Chang KJ. Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) in the USA (abstract). Endoscopy 1998;30:A159-60. [ Links ] 2. Rösch T. Endoscopic ultrasonography. British Journal of Surgery 1997;84:1329-31. [ Links ] 3. Hawes RH. Endoscopic ultrasound. Gastrointest Endosc Clin N Am 2000;10:161-74. [ Links ] 4. Erickson RA. EUS-guided FNA. Gastrointest Endosc 2004;60:267-79. [ Links ] 5. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [ Links ] 6. Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc 1997;45:243-50. [ Links ] 7. Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med 2001;134:459-64. [ Links ] 8. Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc 2001;53:485-91. [ Links ] 9. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc 2000;51:184-90. [ Links ] 10. Mesa H, Stelow EB, Stanley MW, Mallery S, Lai R, Bardales RH. Diagnosis of non-primary pancreatic neoplasms by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol 2004;31:313-8. [ Links ] 11. Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 1986;2:165-73. [ Links ] 12. Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology 1993;187:99-104. [ Links ] 13. Welch TJ, Sheedy PF 2nd, Johnson CD, Johnson CM, Stephens DH. CT-guided biopsy: Prospective analysis of 1,000 procedures. Radiology 1989;171:493-6. [ Links ] 14. Harrison BD, Thorpe RS, Kitchener PG, McCann BG, Pilling JR. Percutaneous Trucut lung biopsy in the diagnosis of localized pulmonary lesions. Thorax 1984;39:493-9. [ Links ] 15. Ingram DM, Sheiner HJ, Shilkin KB. Operative biopsy of the pancreas using the Trucut needle. Aust N Z J Surg 1978;48:203-6. [ Links ] 16. Zinzani PL, Colecchia A, Festi D, Magagnoli M, Larocca A, Ascani S, et al. Ultrasound-guided core-needle biopsy is effective in the initial diagnosis of lymphoma patients. Haematologica 1998;83:989-92. [ Links ] 17. Radhakrishnan V. Transrectal Tru-cut biopsy of the prostate using a protective sheath. Br J Urol 1994;73:454. [ Links ] 18. Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: A prospective study and review of the literature. J Urol 1998;160:2115-20. [ Links ] 19. Durup Scheel-Hincke J, Mortensen MB, Pless T, Hovendal CP. Laparoscopic four-way ultrasound probe with histologic biopsy facility using a flexible Tru-cut needle. Surg Endosc 2000;14:867-9. [ Links ] 20. Indinnimeo M, Cicchini C, Stazi A, Mingazzini P, Ghini C, Pavone P. Trans anal full thickness Tru-cut needle biopsies in anal canal tumors after conservative treatment. Oncol Rep 1998;5:325-7. [ Links ] 21. Sada PN, Ramakrishna B, Thomas CP, Govil S, Koshi T, Chandy G. Transjugular liver biopsy: a comparison of aspiration and trucut techniques. Liver 1997;17:257-9. [ Links ] 22. Chau TN, Tong SW, Li TM, To HT, Lee KC, Lai JY, et al. Transjugular liver biopsy with an automated Trucut-type needle: comparative study with percutaneous liver biopsy. Eur J Gastroenterol Hepatol 2002;14:19-24. [ Links ] 23. Nyman RS, Cappelen-Smith J, Brismar J, von Sinner W, Kagevi I. Yield and complications in ultrasound-guided biopsy of abdominal lesions. Comparison of fine-needle aspiration biopsy and 1.2-mm needle core biopsy using an automated biopsy gun. Acta Radiologica 1995;36:485-90. [ Links ] 24. Bocking A, Klose KC, Kyll HJ, Hauptmann S. Cytologic versus histologic evaluation of needle biopsy of the lung, hilum and mediastinum. Sensitivity, specificity and typing accuracy. Acta Cytologica 1995;39:463-71. [ Links ] 25. Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, Soehendra N. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc 1998;47:121-7. [ Links ] 26. Harada N, Kouzu T, Arima M, Isono K. Endoscopic ultrasound-guided histologic needle biopsy: Preliminary results using a newly developed endoscopic ultrasound transducer. Gastrointest Endosc 1996;44:327-30. [ Links ] 27. Iglesias-Garcia J, Dominguez-Muñoz JE, Lozano-Leon A, Abdulkader I, Lariño-Noia J, Antunez J, et al. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol 2007;13:289-93. [ Links ] 28. Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy 2006;38:919-24. [ Links ] 29. Larghi, A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc 2011;74:504-10. [ Links ] 30. Stavropoulos SN, Im GY, Jlayer Z, Harris MD, Pitea TC, Turi GK, et al. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc 2012;75:310-8. [ Links ] 31. Wiersema MJ, Levy MJ, Harewood GC, Vazquez-Sequeiros E, Jondal ML, Wiersema LM. Initial experience with EUS-guided Trucut needle biopsy of perigastric organs. Gastrointest Endosc 2002;56:275-8. [ Links ] 32. Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003;57:101-6. [ Links ] 33. Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc 2005;62:417-26. [ Links ] 34. Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Practice & Research Clinical Gastroenterology 2009;23:743-59. [ Links ] 35. Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: A prospective study. Gastrointest Endosc 2004;59:185-90. [ Links ] 36. Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, et al. Comparison of EUS guided 19-gauge Trucut needle biopsy with EUS-guided fine needle aspiration. Endoscopy 2004;36:397-401. [ Links ] 37. Wahnschaffe U, Ullrich R, Mayerle J, Lerch MM, Zeitz M, Faiss S. EUS-guided Trucut needle biopsies as first-line diagnostic method for patients with intestinal or extraintestinal mass lesions. Surg Endosc 2009;23:2351-5. [ Links ] 38. Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: A large tertiary referral center experience. Am J Gastroenterol 2009;104:584-91. [ Links ] 39. Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, et al. Feasibility and yield of a new EUS-histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc 2011;73:1189-96. [ Links ] 40. Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: A cytopathologist's perspective. Am J Clin Pathol 2003;120:351-67. [ Links ] 41. Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine needle aspiration: Sampling, pitfalls and quality management. Clinical Gastroenterol Hepatol 2007;5:1248-54. [ Links ] 42. Iglesias-Garcia J, Dominguez-Muñoz JE, Abdulkader I, Lariño-Noia J, Eugenyeva E, Lozano-Leon A, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011;106:1705-10. [ Links ] 43. Williams DB, Sahai AV, Aabakken L, Penma ID, van Velse A, Webb J, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single center experience. Gut 1999;44:720-6. [ Links ] 44. Saftiou A, Villman P, Guldhammer SB, Georgescu CV. Endoscopic ultrasound (EUS)-guided trucut biopsy add significant information to EUS-fine needle aspiration in selected patients: A prospective study. Scandivanian J Gastroenterol 2007;42:117-25. [ Links ] 45. Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: A prospective study. Cytopathology 2006;17:27-33. [ Links ] 46. Storch I, Shah M, Turner R, Donna E, Ribeiro A. Endoscopic ultrasound fine needle aspiration and trucut biopsy in thoracic lesions: When tissue is the issue. Surg Endosc 2008;22:86-90. [ Links ] 47. Aithal GP, Anagnostopoulos GK, Tam W, Dean J, Zaitoun A, Kocjan G, et al. EUS-guided tissue sampling: Comparison of "dual sampling" (trucut biopsy plus FNA) with "sequential sampling" (trucut biopsy and then FNA as required). Endoscopy 2007;39:725-30. [ Links ] 48. Adler DG, Jacobson BC, Davila RE, Hirota WK, Leighton JA, Qureshi WA, et al. ASGE guideline: Complications of EUS. Gastrointest Endosc 2005;61:8-12. [ Links ] 49. Eloubeidi MA, Tamhane A, Varadajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic mass lesions. Gastrointest Endosc 2006;63:622-9. [ Links ] Received: 15-07-2013 ![]() Correspondence:
Correspondence:
Julio Iglesias-García
Gastroenterology Department
Hospital Universitario de Santiago de Compostela
c/ Choupana, s/n.
15706 Santiago de Compostela
A Coruña, Spain
e-mail: julio.iglesias.garcia@sergas.es
Accepted: 09-01-2014