Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.11 Madrid nov. 2016
https://dx.doi.org/10.17235/reed.2016.4058/2016
REVIEW
Mechanisms responsible for neuromuscular relaxation in the gastrointestinal tract
Mecanismos responsables de la relajación neuromuscular en el tracto gastrointestinal
Diana Gallego1,2, Noemí Mañé1, Víctor Gil1, Miriam Martínez-Cutillas1 and Marcel Jiménez1,2
1Department of Cellular Biology, Physiology and Immunology and Instituto de Neurociencias. Universitat Autònoma de Barcelona. Barcelona, Spain.
2Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). Instituto de Salud Carlos III. Barcelona, Spain
Diana Gallego receives support from CIBERehd (Instituto de Salud Carlos III).
ABSTRACT
The enteric nervous system (ENS) is responsible for the genesis of motor patterns ensuring an appropriate intestinal transit. Enteric motor neurons are classified into afferent neurons, interneurons and motorneurons. Motorneurons are excitatory or inhibitory causing smooth muscle contraction and relaxation respectively. Muscle relaxation mechanisms are key for the understanding of physiological processes such as sphincter relaxation, gastric accommodation, or the descending phase of the peristaltic reflex. Nitric oxide (NO) and ATP or a related purine are the primary inhibitory neurotransmitters. Nitrergic neurons synthesize NO through nNOS enzyme activity. NO diffuses across the cell membrane to bind guanylyl cyclase, and then activates a number of intracellular mechanisms that ultimately result in muscle relaxation. ATP is an inhibitory neurotransmitter together with NO. The P2Y1 receptor has been identified as a the purine receptor responsible for smooth muscle relaxation. Although, probably, no clinician doubts about the significance of NO in the pathophysiology of gastrointestinal motility, the relevance of purinergic neurotransmission is apparently much lower, as ATP has not been associated with any specific motor dysfunction yet. The goal of this review is to discuss the function of both relaxation mechanisms in order to establish the physiological grounds of potential motor dysfunctions arising from impaired intestinal relaxation.
Key words: Enteric nervous system. Inhibitory neurotransmission. Nitric oxide. ATP. P2Y1 receptors.
RESUMEN
El sistema nervioso entérico (SNE) es responsable de la génesis de los patrones motores que aseguran un correcto tránsito intestinal. Las neuronas entéricas se clasifican en aferentes, interneuronas y motoneuronas, que pueden a su vez ser excitatorias, causando contracción, o inhibitorias, provocando la relajación de la musculatura lisa. Los mecanismos de relajación muscular son claves para entender procesos fisiológicos como la relajación de los esfínteres, la acomodación gástrica o la fase descendente del reflejo peristáltico. El óxido nítrico (NO) y el ATP o una purina relacionada son los principales neurotransmisores inhibitorios. Las neuronas nitrérgicas sintetizan NO a partir del enzima nNOS. El NO difunde a través de la membrana celular uniéndose a su receptor, la guanilil ciclasa, y activando posteriormente una serie de mecanismos intracelulares que provocan finalmente una relajación muscular. El ATP actúa como neurotransmisor inhibitorio junto con el NO y el receptor de membrana purinérgico P2Y1 ha sido identificado como elemento clave para entender cómo el ATP relaja la musculatura intestinal. Aunque probablemente ningún clínico duda de la importancia del NO en la fisiopatología motora digestiva, la relevancia de la neurotransmisión purinérgica es aparentemente mucho menor puesto que el ATP no ha sido todavía asociado a una disfunción motora concreta. El objetivo de esta revisión es mostrar el funcionamiento de ambos mecanismos de relajación para poder establecer las bases fisiológicas de posibles disfunciones motoras asociadas a la alteración de la relajación intestinal.
Palabras clave: Sistema nervioso entérico. Neurotransmisión inhibitoria. Óxido nítrico. ATP. Receptores P2Y1.
Introduction
The gastrointestinal (GI) tract includes 100 million neurons, which make up the enteric nervous system (ENS); these neurons are spread throughout the digestive tract, and constitute two plexuses: submucosal plexus (or Meissner's plexus) and myenteric plexus (or Auerbach's plexus). The myenteric plexus is located between the circular and longitudinal muscle layers, and runs from the esophagus to the anal canal. Its primary role is the regulation of motor function, but it might also be involved in secretion. The submucosal plexus lies beneath the muscularis mucosae, and supplies innervation to the mucosa. The ENS, together with the interstitial cells of Cajal (ICCs), is responsible for the regulation of mixing and propulsion movements in the GI tract, with smooth muscle being its ultimate effector. The ENS is remarkably independent despite being influenced by the central nervous system through afferent and efferent pathways from the autonomic nervous system.
ENS neurons may be classified according to their function as afferent neurons, interneurons, and motoneurons (1-4). Intrinsic primary afferent neurons (IPANs) have their cell bodies both in myenteric and submucosal plexus ganglia, and collect "sensory" innervation from nerve fibers projecting to the intestinal mucosa. IPANs respond to chemical stimuli and mechanical mucosal deformation, as well as to radial stretching and muscle tension. The mucosa also harbors enterochromaffin cells, which release mediators such as serotonin and ATP (5), and respond to luminal stimuli, which in turn activate IPAN terminals (3,6). The activation of these cells is a first step in the triggering of motor reflexes, as they translate stimuli from the intestinal lumen into nerve impulses that are transmitted to interneurons and motor neurons.
Interneurons form chains running both orally and aborally, making up circuits within the myenteric plexus. Interneurons may therefore be classified as ascending (with oral projections) or descending (with aboral projections). Interneurons participate in the polarity of the persitaltic reflex. Mucosal stimulation releases mediators (serotonin and ATP) that activate IPANs, which in turn activate interneurons. These interneurons orally activate excitatory motoneurons, thus resulting in smooth muscle contraction, and they aborally activate inhibitory motoneurons, which results in smooth muscle relaxation and facilitates bolus propulsion in the peristaltic direction (1,7,8).
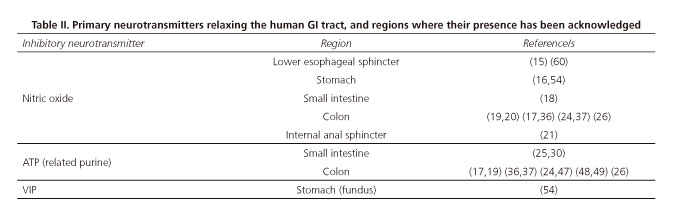
Motoneurons represent the final connection with smooth muscle cells in the circular and longitudinal layers. They may be classified into excitatory and inhibitory motoneurons according to the neurotransmitters they code for (2). Excitatory motoneurons release acetylcholine (ACh) and tachykinins (mainly NKA and substance P). In contrast, inhibitory motoneurons release nitric oxide (NO) and ATP, but may also release other neuromodulators such as VIP, PACAP, carbon monoxide (CO), and hydrogen sulfide (H2S) (9,10), although in these cases functional evidences are less clear.
This review focuses in the mechanims responsible for neuromuscular relaxation. In particular we will address receptors and signalling pathways involved in neural mediated relaxation. Furthermore, it attempts to summarise its significance for optimal digestive functioning as well as its role in GI motility disorders.
In vitro Study Methods
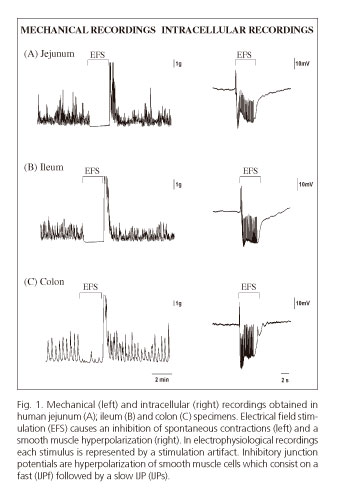
The organ bath and the microeletrode technique are usually employed to study neural mediated inhibitory responses in vitro (Fig. 1.) Transmural biopsy samples obtained from surgical procedures can be studied with these two experimental procedures. Factors such as post-operative time, medication, anesthetics, and underlying pathological conditions should always be considered for an appropriate interpretation of results (11). Another approach is the use of tissue from laboratory animals. Genetically modified mice have been a crucial biological tool for the understanding of receptors and signaling pathways involved in gastrointestinal relaxation (12,13). Laboratory animals are usefull to develop models of disease mimicking human motor dysfunction.
These techniques allow a functional assessment of the various elements involved in motility regulation (enteric neurons, ICCs, smooth muscle). The mechanical recordings described in figure 1 show the spontaneous contractility of transmural preparations from the small bowel (jejunum and ileum) and the colon. When a preparation is electrically stimulated, an action potential is generated by motoneurons that results in the release of inhibitory neurotransmitters causing a cessation of spontaneous contractions. This mechanical inhibition correlates with smooth muscle hyperpolarization observed in intracellular recordings obtained with microelectrodes. Such hyperpolarization is called an inhibitory junction potential (IJP). Hyperpolarization represents a negative increase in membrane potential that moves muscle cells away from the voltage required for the opening of voltage-dependent calcium channels (Cav), which translates into mechanical relaxation. In most species and areas of the GI tract, the IJP has two phases an initial IJPf (IJPfast) followed by a second, more sustained hyperpolarization phase called IJPs (IJP slow).
Major ens inhibitory neurotransmitters
Nitric Oxide (NO)
NO was first reported in the 1990s as a major inhibitory neurotransmitter in the GI tract (14). NO mediates relaxation in several areas of the GI tract, including the esophageal sphincter (15), stomach (promoting gastric accommodation and emptying) (16), small and large bowel (17-20), and internal anal sphincter (21). It is presently the most widely understood inhibitory neurotransmitter given its role in pathological conditions (22).
NO is a molecule generated by NO-synthases (NOS), which produce NO from L-arginine. Three independent genes code for all three NOS isoforms, namely, NOS-1, NOS-2 and NOS-3; these genes code for the neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) isoenzymes, respectively. All of them produce NO via independent mechanisms. The primary source of NO in GI neurons is nNOS. During inflammation NO is overproduced in different cell structures through iNOS. nNOS inhibition with L-NNA blocks the IJPs, thus revealing its nitrergic origin. NO overproduction may result in excessive intestinal relaxation, as seen in some motor dysfunctions (22).
Receptor and Intracellular Pathway
NO is a lipophilic compound that diffuses freely across plasma membranes. The most widely characterized intracellular pathway for NO is the one mediated by cytoplasmic soluble guanylyl cyclase (sGC), which produces cyclic GMP (23). This in turn activates a protein kinase G (PKG) to generate a phosphorylation cascade that ultimately activates myosin light chain phosphatase, thus relaxing smooth muscle cells. PKG also activates potassium channels. Their opening results in hyperpolarization from potassium efflux from smooth muscle cells. Chloride channel closure has also been proposed as a mechanism causing smooth muscle hpierpolarization (13). Because of increased negativity within the smooth muscle cell, relaxation ensues in response to NO (13,23). Phosphodiesterase 5 (PDE5) causes in cGMP degradation. PDE5 inhibitors such as sildenafil have been suggested for the management of GI conditions with impaired nitrergic pathway. From a research standpoint, sGC blockade with ODQ as well as with L-NNA, an inhibitor of NO synthase, results in IJP inhibition. This hyperpolarization is responsible for sustained mechanical relaxation. Furthermore, NO is tonically released, and is held responsible for the so-called inhibitory inhibitory neural tone (24-26).
ATP OR Related Nucleotide
Early studies
In 1970, ATP (or a related nucleotide) was posited by Burnstock and colleagues as an inhibitory neurotransmitter in the GI tract. At that time the result was very controversial since it was not easy to accept that the main energy molecule produced in the mitochondria it was also a chemical neurotransmitter. At present we know that ATP is released by inhibitory neurons and relaxes smooth muscle, and "purinergic" neurons have been identified with the quinacrine technique (27,28). This technique labels vesicles with high ATP contents, which probably does not guarantee their being exclusively purinergic. Data obtained from the human small and large bowel, and from a number of laboratory animals, show that ATP would be responsible for initial fast hyperpolarization or IJPf (17,19,29,30). The IJPf is responsible for phasic relaxations since it undergoes a rundown phenomenom, that is, successive stimuli result in decreasing responses. Accordingly the IJPf cannot be mantained over time and can not cause sustained relaxations (24,26).
P2Y1 Receptor identified as responsible for bowel relaxation
Identifying the purinergic receptor involved in intestinal hyperpolarization and relaxation is mandatory to reveal the pathophysiological mechanisms associated with this pathway. However, several factors, including the lack of selective antagonist for each receptor subtype (see below), have for long hindered the identification of the purine receptor involved in smooth muscle relaxation. Two purine receptors (P1 and P2) have been reported. P1 receptors are adenosine receptors, and four subtypes have been identified: A1, A2A, A2B, and A3. All of them are coupled to a G protein, which activates second messengers. They stimulate (A1, A3) or inhibit (A2A, A2B) adenylate cyclase. P2 receptors mainly recognize ATP, ADP, UDP, and UTP. Within this family two receptor subtypes (P2X and P2Y) are included. P2X receptors are ionotropic receptors, that is, ion channels that mediate cation influx when activated. Seven P2X receptor subtypes are known (P2X1-P2X7). P2Y receptors are metabotropic receptors, and eight subtypes have been described: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14, although newer subtypes such as P2Y15 have been suggested of late. Most are coupled to a G protein that activates phospholipase C; this results in diacylglycerol (DAG) and phosphatidylinositol trisphosphate (IP3), which mediated calcium release from intracellular stores. Some receptors may be connected to G proteins that, in turn, activate adenylate cyclase, thus increasing cyclic AMP (31;32) (Table I). Purinergic receptors are present in numerous cell types since purines play a role in many cell communication mechanims, including signal transduction at the epithelial level, interneuronal communication, afferent activation, neuroglial communication, etc. (33). The question that we have tried to answer during the last few years is: Which one of these receptors is responsible for intestinal relaxation?
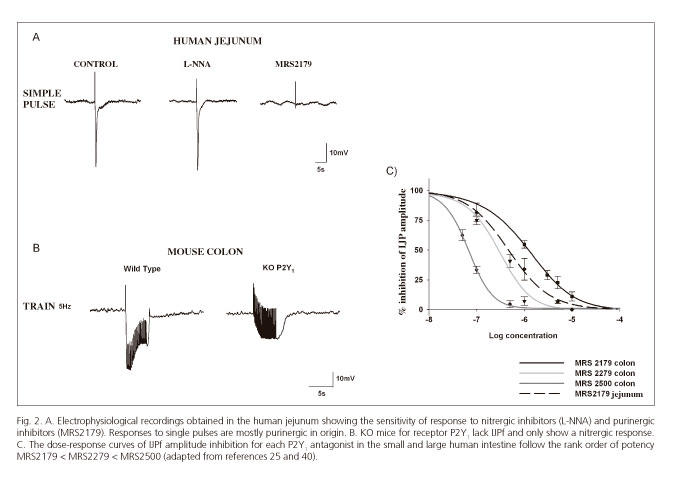
Suramin and PPADs are scarcely selective purinergic antagonists that do not allow differentiation between distinct P2 receptors. The development of specific antagonists such as, for instance MRS2179, which blocks P2Y1 receptors (34;35), allowed to identify pharmacologically that ATP, acts post-junctionally through P2Y1 receptors causing smooth muscle relaxation in several GI tract areas (12,24,25,36,37). Subsequently, two new antagonists (MRS2279 and MRS2500) with greater affinity for the P2Y1 receptor, (35,38,39) confirmed the crucial role of this receptor subtype in purinergic neurotransmission. According to pharmacological data the rank of order of potency was: MRS2179 < MRS2279 < MRS2500 (40) (Fig. 2). These results have been recently confirmed in genetically modified animals (knockout mice) for the P2Y1 receptor (41-43). These knockouts exhibit neither the purinergic IJP, nor the purinergic component of relaxation (Fig. 2).
The P2Y1 receptor is responsible for mediating relaxation in several areas of the GI tract (12). P2Y1 receptors are coupled to a G protein (Gq) that activates phospholipase C. The latter hydrolyzes a membrane lipid to provide two second messengers, DAG and IP2, which causes calcium influx from the sarcoplasmic reticulum (44,45). Calcium activates small conductance calcium activated potassium channels sK(Ca) resulting in potassium efflux leading smooth muscle hyperpolarization and relaxation.
Figure 2 shows the electrophysiological response to a single pulse in the human jejunum, which is similar to other GI portions (ileum and colon). Except for the esophageal sphincter, where these responses are purely nitrergic, the hyperpolarization seen both in the small bowel and colon is mainly purinergic in nature.
ATP and maybe other neurotransmitters?
Whether ATP or a related nucleotide is the major purinergic neurotransmitter is presently a subject of debate. ATP would be released at the neuromuscular junction, and various metabolites (ADP, AMP, adenosine) would result from the action of ectonucleotidases (breakdown enzymes). Each of these has greater or lesser affinity for the different purine receptors subtypes (31). It was recently hypothesized that β-NAD (β-nicotinamide adenine dinucleotide), ADPR (ADP ribose) or Up4A (uridine adenosine tetraphosphate) might be (even instead of ATP) the purinergic neurotransmitter in the GI tract (46-49). However, some experimental data have nuanced these results (43,50-52).
Other Neurotransmitters/Neuromodulators
Other compounds are possible inhibitory neurotransmitters in the human GI tract. Peptides such as VIP and PACAP (53) would play a role in the relaxation of some areas, including the gastric fundus and colon (54,55). Also gases such as CO (56,57) or H2S (58,59) are potential inhibitory neurotransmitters or neuromodulators. However, functional data demonstrating that these neurotransmitters do play a role in muscle relaxation remain inconsistent. Table II summarizes the most significant in vitro studies and the human GI tract portions where these inhibitory neurotransmitters have been reported.
Functional NO-ATP co-transmission
The development of specific P2Y1 antagonists (35,40) has allowed the isolation of both the purinergic and nitrergic components of inhibitory neurotransmission (24,26). Currently, none of the available ATP-labeling techniques ensure 100% reliability in the identification of purinergic neurons. Therefore, co-transmission, that is, the release of both neurotransmitters by the same neuron, remains to be demonstrated. However, co-transmission is assumed since nobody has ever found a dual inhibitory innervation. It is important to identify which differential parameters enhance the release of each transmitter, and their action on the post-junction cell. Based on experimental findings, we may say that these two neurotransmitters have complementary functions regarding GI tract relaxation. As discussed above NO is necessary for gastric acomodation. In contrast. In contrast ATP may have a key role in transient relaxation as is, for instance, the case with the descending phase of the peristaltic reflex (24,26), and would therefore predominate in areas with more relevant peristalsis, including the small intestine and colon (17,19,24,25,30,36,37).
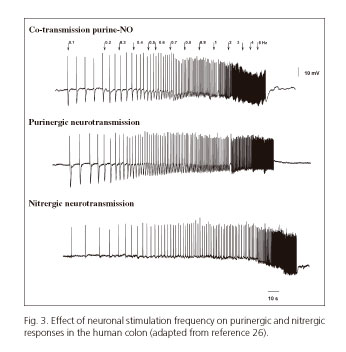
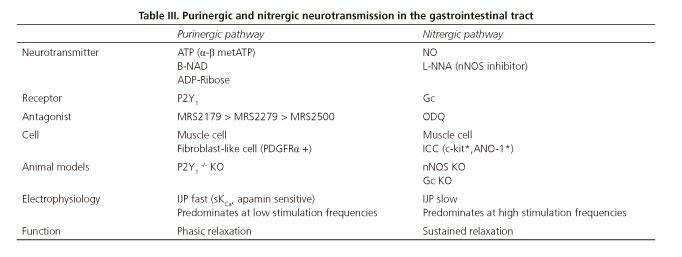
Recent studies from our laboratory show that the effect of both neurotransmitters depends on the frequency of nerve stimulation of the preparation (24-26). At low frequencies (below 1 Hz) purinergic response predominates, whereas higher frequencies attenuate purinergic responses and increase the nitrergic ones (26) (Fig. 3). This allowed us to develop a number of mathematical models that relate the response obtained with the frequency of stimulation. The frequency of stimulation can be possibly associated to the endogenous firing frequency of a group of motoneurons. According to experimental data higher neuronal firing rates would result in sustained relaxation (with NO release), and lower firing rates would result in big though transient relaxation (with ATP release) (26,63,64). If our hypothesis is true, one neuron could play different functions according to its firing rate. In samples from animal models, higher-frequency stimuli may result in the release of other neurotransmitters such as VIP (65). However this has still been not confirmed in human tissue. Table III details the characteristics of the two primary inhibitory (i.e., purinergic and nitrergic) pathways in the GI tract.
Direct VS Indirect Action
A hot topic currently debated is whether inhibitory neurotransmitters (NO and ATP) act directly via the neuromuscular junction or through an intermediate cell located between motoneurons and the smooth muscle. ICCs possibly mediate nitrergic neuromuscular transmission (66,67) whereas purinergic neuromuscular transmission is possibly mediated by platelet-derived growth factor receptor α positive cells (PDGRF α) (68-70). Mutant animals with impaired ICC development do have inhibitory nitrergic neurotransmission supporting a direct action of NO on smooth muscle (71,72). Recent studies have shown that both ICCs and smooth muscle cells may mediate the effects of NO (73). The role of PDGRFα-positive cells as mediators of purinergic relaxation remains uncertain since no studies with animals lacking this cell subtype are yet available. Studies advocating for the alternate indirect hypothesis are based on morphological argumentation. Interstitial cells are highly innervated and interspersed between nerve terminals and smooth muscle cells (74). In addition, interstitial cells have receptors and mediators for these neurotransmitters (75), which render the response of isolated cells to the exogenous addition of agonists higher than that of smooth muscle (70). In experimental models lacking ICCs nitrergic neurotransmission is absent (76-78). Figure 4 shows both GI tract neurotransmission hypotheses.
The development of conditional knockouts deficient in receptors/pathways for some cell subtypes opens up multiple research possibilities in this setting. An example is the above-mentioned study by Lies et al. (73), carried out in a mouse deficient for sGC (the receptor mediating NO relaxation) only in ICCs or only in smooth muscle cells. Their findings showed that loss of this receptor in ICCs greatly reduced neurotransmission (hyperpolarization or IJPs), but a functional portion remained, which suggests that both hypotheses might be compatible (partly direct and partly indirect).
Digestive conditions associated with inhibitory neurotransmission
Changes in the pathways leading to gut relaxation have been associated with a significant number of motor digestive disorders. However, the available clinical data on the relevance of these neurotransmitters for relaxation varies amongst the various motor disorders that are listed below.
Absence of NO and neurons of the lower esophageal sphincter has been reported in patients with achalasia (79). A recent study in children from one family reveals that changes in the gene coding for nNOS (NOS-1) results in pediatric achalasia (80). The use of NO breakdown inhibitors such as sildenafil (a PDE5 inhibitor) is a drug therapy approach potentially useful for some of these patients (80-82). In the anal sphincter, NO induces relaxation as shown, for instance, by the efficacy of chronic anal fissure management with topical nitrites, which act as NO donors and thus favor healing by reducing sphincter tone. In these two conditions NO plays a highly relevant role.
Gastric emptying requires appropriate fundus accommodation and rhythmic antral contractions in order to empty both liquids and solids. Gastric accommodation is based on fundic relaxation, and studies in humans have shown such relaxation to be dependent upon NO (83,84). Gastroparesia is defined as slow gastric emptying with no apparent obstruction, and is usually associated with diabetes or it is considered idipoathic. Loss of nNOS in animals has been related to diabetic gastrointestinal disease (85,86). Changes in ICCs and nitrergic neurons have been recently described in patients with either idiopathic (40%) or diabetic (20%) gastroparesis (87). Again, sildenafil has been proposed as a pharmacological for the relaxation of the proximal stomach (88). nNOS deficiency together with loss of ICCs has been associated with colonic inertia and constipation resulting from diabetic enteropathy (89,90). Samples from patients during asymptomatic diverticular disease show increased nNOS expression and NO production (91). Similar findings have been described in experimental model of irritable bowel syndrome (IBS) (92). Importantly, in some of these conditions, e.g., in diabetic neuropathy or IBS, extrinsic autonomic innervation, which includes both afferent and efferent pathways, may also be involved. In inflammation, the overproduction of NO from inducible iNOS may be responsible for excessive smooth muscle relaxation (93,94). However, further studies are needed to elucidate whether NO is directly responsible for motor changes (as supported, for instance, by topical nitrite effectiveness in the management of chronic anal fissure, where nitrites act as NO donors thus favoring healing by reducing sphincter tone) or is an epiphenomenon, and motor changes result from other inflammation mediators capable of inducing muscle relaxation (95).
What role does ATP play in GI tract disorders? This question is much harder to answer since, despite the identification of its physiological function, the role of ATP in motor disorders has been much less researched. However, some clues do suggest that ATP, just as NO, may play a role in gastrointestinal motor dysfunction.
A major limitation in attempting to answer the above question is our lack of markers for purinergic neurons. Simply put, a clinician cannot ask a pathologist whether purinergic neurons are present in a transmural biopsy sample. In fact, in spite of everyone assumes a co-transmission phenomenon, that is, that one same neuron is simultaneously nitrergic and purinergic, nobody has ever shown the co-localization of both neurotransmitters. Some advances have been currently made in this field, and markers have been suggested for ATP-containing neural vesicles (96), although their specificity could not be demonstrated since many vesicles may contain ATP.
During inflammation, multiple inflammatory cells, as well as necrotic cells, release nucleotides into the extracellular space (97). These nucleotides may have an effect both at the pre-synaptic and post-synaptic level. Some purines can act pre-synaptically to inhibit ATP release in the human jejunum (52). These nucleotides may also play a role at the post-synaptic level by desensitizing the P2Y1 receptor, as has been shown in the human small bowel and colon (24-26,30,36). Experimental models of colitis showing a decreased purinergic IJP component (IJPf) confirm this hypothesis. This desensitization or decreased IJPf may have a significant impact on intestinal motility; in knockout mice for the receptor mediating purinergic relaxation (P2Y1) the absence of IJPf translates into a severely delayed colonic transit. Possibly, ATP also plays a role in many of the motor disorders described for NO where the ENS is involved. Importantly in our view, the role of ATP/P2Y1 as inhibitory neurotransmitter in conditions such as achalasia, gastroparesis, intestinal pseudo-obstruction or colonic inertia deserves further study. Preliminary data obtained in our laboratory show, for instance, lack of purinergic neurotransmission in samples from transition zones in Hirschsprung's disease (98).
Hopefully, we have convinced the reader that ATP is key for the understanding of intestinal relaxation. Our goal was to identify the P2Y1 receptor as a key player in purinergic transduction. We have therefore identified a new pharmacological target potentially useful in the management of GI motor dysfunction. Future lines of research include the study of genetic mutations able to compromise purinergic neuromuscular transmission. Another important issue is to count number and structual changes in PDGFRα+ cells from specimens from subjects with GI motor disorders. It is also essential the design of animal models with conditional deletion of receptors in specific cells. All these studies should be undertaken to gain insight into this signaling pathway, and to expand the resulting knowledge to motor pathophysiology regarding the GI tract.
References
1. Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 1999;61:117-42. DOI: 10.1146/annurev.physiol.61.1.117. [ Links ]
2. Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 2000;81:87-96. DOI: 10.1016/S0165-1838(00)00127-2. [ Links ]
3. Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 2000;47(Suppl. 4):iv15-iv19. DOI: 10.1136/gut.47.suppl_4.iv15. [ Links ]
4. Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 2001;262:58-70. DOI: 10.1002/1097-0185(20010101) 262:1<58::AID-AR1011>3.0.CO;2-V. [ Links ]
5. Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: Activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci 2002;22:4767-75. [ Links ]
6. Furness JB, Jones C, Nurgali K, et al. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 2004;72:143-64. DOI: 10.1016/j.pneurobio.2003.12.004. [ Links ]
7. Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 2004;16(Suppl. 1):34-8. DOI: 10.1111/j.1743-3150.2004.00472.x. [ Links ]
8. Hansen MB. The enteric nervous system I: Organisation and classification. Pharmacol Toxicol 2003;92:105-13. DOI: 10.1034/j.1600-0773.2003.t01-1-920301.x. [ Links ]
9. Lecci A, Santicioli P, Maggi CA. Pharmacology of transmission to gastrointestinal muscle. Curr Opin Pharmacol 2002;2:630-41. DOI: 10.1016/S1471-4892(02)00225-4. [ Links ]
10. Linden DR, Levitt MD, Farrugia G, et al. Endogenous production of H2S in the gastrointestinal tract: Still in search of a physiologic function. Antioxid Redox Signal 2010;12:1135-46. DOI: 10.1089/ars.2009.2885. [ Links ]
11. Sanger GJ, Broad J, Kung V, et al. Translational neuropharmacology: The use of human isolated gastrointestinal tissues. Br J Pharmacol 2013;168:28-43. DOI: 10.1111/j.1476-5381.2012.02198.x. [ Links ]
12. Jiménez M, Clave P, Accarino A, et al. Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol 2014;171:4360-75. DOI: 10.1111/bph.12802. [ Links ]
13. Sanders KM, Koh SD, Ro S, et al. Regulation of gastrointestinal motility - Insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 2012;9:633-45. DOI: 10.1038/nrgastro.2012.168. [ Links ]
14. Bult H, Boeckxstaens GE, Pelckmans PA, et al. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 1990;345:346-7. DOI: 10.1038/345346a0. [ Links ]
15. González AA, Farre R, Clave P. Different responsiveness of excitatory and inhibitory enteric motor neurons in the human esophagus to electrical field stimulation and to nicotine. Am J Physiol Gastrointest Liver Physiol 2004;287:G299-306. DOI: 10.1152/ajpgi.00534.2003. [ Links ]
16. Andrews CN, Bharucha AE, Camilleri M, et al. Nitrergic contribution to gastric relaxation induced by glucagon-like peptide-1 (GLP-1) in healthy adults. Am J Physiol Gastrointest Liver Physiol 2007;292:G1359-65. [ Links ]
17. Keef KD, Du C, Ward SM, et al. Enteric inhibitory neural regulation of human colonic circular muscle: Role of nitric oxide. Gastroenterology 1993;105:1009-16. [ Links ]
18. Stark ME, Bauer AJ, Sarr MG, et al. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology 1993;104:398-409. [ Links ]
19. Boeckxstaens GE, Pelckmans PA, Herman AG, et al. Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology 1993;104:690-7. [ Links ]
20. Tam FS, Hillier K. The role of nitric oxide in mediating non-adrenergic non-cholinergic relaxation in longitudinal muscle of human taenia coli. Life Sci 1992;51:1277-84. DOI: 10.1016/0024-3205(92)90017-J. [ Links ]
21. O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: The role of nitric oxide. Gut 1993;34:689-93. DOI: 10.1136/gut.34.5.689. [ Links ]
22. Shah V, Lyford G, Gores G, et al. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004;126:903-13. DOI: 10.1053/j.gastro.2003.11.046. [ Links ]
23. De Man JG, De Winter BY, Herman AG, et al. Study on the cyclic GMP-dependency of relaxations to endogenous and exogenous nitric oxide in the mouse gastrointestinal tract. Br J Pharmacol 2007;150:88-96. DOI: 10.1038/sj.bjp.0706964. [ Links ]
24. Gallego D, Gil V, Aleu J, et al. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol 2008;295:G522-33. DOI: 10.1152/ajpgi.00510.2007. [ Links ]
25. Gallego D, Malagelada C, Accarino A, et al. Nitrergic and purinergic mechanisms evoke inhibitory neuromuscular transmission in the human small intestine. Neurogastroenterol Motil 2014;26:419-29. DOI: 10.1111/nmo.12293. [ Links ]
26. Mane N, Gil V, Martínez-Cutillas M, et al. Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol (Oxf) 2014;212:293-305. DOI: 10.1111/apha.12408. [ Links ]
27. Burnstock G, Campbell G, Satchell D, et al. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol 1970;40:668-88. DOI: 10.1111/j.1476-5381.1970.tb10646.x. [ Links ]
28. Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 2006;27:166-76. DOI: 10.1016/j.tips.2006.01.005. [ Links ]
29. Zagorodnyuk VP, Vladimirova IA, Vovk EV, et al. Studies of the inhibitory non-adrenergic neuromuscular transmission in the smooth muscle of the normal human intestine and from a case of Hirschsprung's disease. J Auton Nerv Syst 1989;26:51-60. DOI: 10.1016/0165-1838(89)90107-0. [ Links ]
30. Xue L, Farrugia G, Sarr MG, et al. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol 1999;276:G1373-9. [ Links ]
31. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413-92. [ Links ]
32. Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 2004;240:31-304. [ Links ]
33. Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 2014;10:3-50. DOI: 10.1007/s11302-013-9397-9. [ Links ]
34. Camaioni E, Boyer JL, Mohanram A, et al. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem 1998;41:183-90. DOI: 10.1021/jm970433l. [ Links ]
35. Cattaneo M, Lecchi A, Ohno M, et al. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol 2004;68:1995-2002. DOI: 10.1016/j.bcp.2004.06.026. [ Links ]
36. Gallego D, Hernández P, Clave P, et al. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol 2006;291:G584-94. DOI: 10.1152/ajpgi.00474.2005. [ Links ]
37. Auli M, Martínez E, Gallego D, et al. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol 2008;155:1043-55. DOI: 10.1038/bjp.2008.332. [ Links ]
38. Kim HS, Barak D, Harden TK, et al. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: Structure-activity relationships and receptor docking. J Med Chem 2001;44:3092-108. DOI: 10.1021/jm010082h. [ Links ]
39. Boyer JL, Adams M, Ravi RG, et al. 2-Chloro N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate is a selective high affinity P2Y(1) receptor antagonist. Br J Pharmacol 2002;135:2004-10. DOI: 10.1038/sj.bjp.0704673. [ Links ]
40. Gallego D, Gil V, Aleu J, et al. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil 2011;23:792-e338. DOI: 10.1111/j.1365-2982.2011.01725.x. [ Links ]
41. Gallego D, Gil V, Martínez-Cutillas M, et al. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol 2012;590:1943-56. DOI: 10.1113/jphysiol.2011.224345. [ Links ]
42. Hwang SJ, Blair PJ, Durnin L, et al. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 2012;590:1957-72. DOI: 10.1113/jphysiol.2011.224634. [ Links ]
43. Gil V, Martínez-Cutillas M, Mane N, et al. P2Y(1) knockout mice lack purinergic neuromuscular transmission in the antrum and cecum. Neurogastroenterol Motil 2013;25:e170-82. DOI: 10.1111/nmo.12060. [ Links ]
44. Hu HZ, Gao N, Zhu MX, et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 2003;550:493-504. DOI: 10.1113/jphysiol.2003.041731. [ Links ]
45. Wood JD. The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol 2006;6:564-70. DOI: 10.1016/j.coph.2006.06.006. [ Links ]
46. Mutafova-Yambolieva VN, Hwang SJ, Hao X, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A 2007;104:16359-64. DOI: 10.1073/pnas.0705510104. [ Links ]
47. Hwang SJ, Durnin L, Dwyer L, et al. Beta-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 2011;140:608-17. DOI: 10.1053/j.gastro.2010.09.039. [ Links ]
48. Durnin L, Hwang SJ, Ward SM, et al. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with beta-NAD(+). J Physiol 2012;590:1921-41. DOI: 10.1113/jphysiol.2011.222414. [ Links ]
49. Durnin L, Hwang SJ, Kurahashi M, et al. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci USA 2014;111:15821-6. DOI: 10.1073/pnas.1409078111. [ Links ]
50. Goyal RK. Evidence for beta-nicotinamide adenine dinucleotide as a purinergic, inhibitory neurotransmitter in doubt. Gastroenterology 2011;141:e27-8. DOI: 10.1053/j.gastro.2011.07.047. [ Links ]
51. Goyal RK, Sullivan MP, Chaudhury A. Progress in understanding of inhibitory purinergic neuromuscular transmission in the gut. Neurogastroenterol Motil 2013;25:203-7. DOI: 10.1111/nmo.12090. [ Links ]
52. Wang GD, Wang XY, Liu S, et al. Beta-nicotinamide adenine dinucleotide acts at prejunctional adenosine A1 receptors to suppress inhibitory musculomotor neurotransmission in guinea pig colon and human jejunum. Am J Physiol Gastrointest Liver Physiol 2015;308:G955-63. DOI: 10.1152/ajpgi.00430.2014. [ Links ]
53. Bitar KN, Makhlouf GM. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal peptide. Science 1982;216:531-3. DOI: 10.1126/science.6176025. [ Links ]
54. Tonini M, De Giorgio R, De Ponti F, et al. Role of nitric oxide- and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. Br J Pharmacol 2000;129:12-20. DOI: 10.1038/sj.bjp.0702977. [ Links ]
55. Schworer H, Clemens A, Katsoulis S, et al. Pituitary adenylate cyclase-activating peptide is a potent modulator of human colonic motility. Scand J Gastroenterol 1993;28:625-32. DOI: 10.3109/ 003655293090 96101. [ Links ]
56. Farrugia G, Miller SM, Rich A, et al. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol 1998;274:G350-8. [ Links ]
57. Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol 2004;556:325-36. DOI: 10.1113/jphysiol.2003.056556. [ Links ]
58. Gallego D, Clave P, Donovan J, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil 2008;20:1306-16. DOI: 10.1111/j.1365-2982.2008.01201.x. [ Links ]
59. Martínez-Cutillas M, Gil V, Mane N, et al. Potential role of the gaseous mediator hydrogen sulphide (H2S) in inhibition of human colonic contractility. Pharmacol Res 2015;93:52-63. DOI: 10.1016/j.phrs.2015.01.002. [ Links ]
60. Lecea B, Gallego D, Farre R, et al. Regional functional specialization and inhibitory nitrergic and nonnitrergic coneurotransmission in the human esophagus. Am J Physiol Gastrointest Liver Physiol 2011;300:G782-94. DOI: 10.1152/ajpgi.00514.2009. [ Links ]
61. Broad J, Hughes F, Chin-Aleong J, et al. Regionally dependent neuromuscular functions of motilin and 5-HT(4) receptors in human isolated esophageal body and gastric fundus. Neurogastroenterol Motil 2014;26:1311-22. DOI: 10.1111/nmo.12394. [ Links ]
62. Broad J, Goralczyk A, Mannur K, et al. Drugs acting at 5-HT4, D2, motilin, and ghrelin receptors differ markedly in how they affect neuromuscular functions in human isolated stomach. Neurogastroenterol Motil 2014;26:851-61. DOI: 10.1111/nmo.12338. [ Links ]
63. Mane N, Gil V, Martínez-Cutillas M, et al. Dynamics of inhibitory co-transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon. Pflugers Arch 2014;466:2305-21. DOI: 10.1007/s00424-014-1500-8. [ Links ]
64. Mane N, Viais R, Martínez-Cutillas M, et al. Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiol (Oxf). 2016;216:120-31. DOI: 10.1111/apha.12599. [ Links ]
65. Keef KD, Saxton SN, McDowall RA, et al. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J Physiol 2013;591:1489-1506. DOI: 10.1113/jphysiol.2012.247684. [ Links ]
66. Ward SM, Sanders KM. Interstitial cells of Cajal: Primary targets of enteric motor innervation. Anat Rec 2001;262:125-35. DOI: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [ Links ]
67. Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996;111:492-515. DOI: 10.1053/gast.1996.v111.pm8690216. [ Links ]
68. Kurahashi M, Nakano Y, Hennig GW, et al. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med 2012;16:1397-404. DOI: 10.1111/j.1582-4934.2011.01510.x. [ Links ]
69. Kurahashi M, Zheng H, Dwyer L, et al. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles. J Physiol 2011;589:697-710. DOI: 10.1113/jphysiol.2010.201129. [ Links ]
70. Kurahashi M, Mutafova-Yambolieva V, Koh SD, et al. Platelet-derived growth factor receptor-alpha-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 2014;307:C561-70. DOI: 10.1152/ajpcell.00080.2014. [ Links ]
71. De Lorijn F, De Jonge WJ, Wedel T, et al. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 2005;54:1107-13. DOI: 10.1136/gut.2004.051045. [ Links ]
72. Terauchi A, Kobayashi D, Mashimo H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 2005;289:G291-9. DOI: 10.1152/ajpgi.00005.2005. [ Links ]
73. Lies B, Gil V, Groneberg D, et al. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2014;307:G98-106. DOI: 10.1152/ajpgi.00082.2014. [ Links ]
74. Ward SM, Sanders KM. Interstitial cells of Cajal: Primary targets of enteric motor innervation. Anat Rec 2001;262:125-35. DOI: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [ Links ]
75. Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRalpha-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil 2013;25:e609-20. DOI: 10.1111/nmo.12174. [ Links ]
76. Burns AJ, Lomax AE, Torihashi S, et al. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 1996;93:12008-13. DOI: 10.1073/pnas.93.21.12008. [ Links ]
77. Ward SM, Morris G, Reese L, et al. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology 1998;115:314-29. DOI: 10.1016/S0016-5085(98)70198-2. [ Links ]
78. Suzuki H, Ward SM, Bayguinov YR, et al. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol 2003;546:751-63. DOI: 10.1113/jphysiol.2002.033365. [ Links ]
79. Mearin F, Papo M, Malagelada JR. Impaired gastric relaxation in patients with achalasia. Gut 1995;36:363-8. DOI: 10.1136/gut.36.3.363. [ Links ]
80. Shteyer E, Edvardson S, Wynia-Smith SL, et al. Truncating mutation in the nitric oxide synthase 1 gene is associated with infantile achalasia. Gastroenterology 2015;148:533-6. DOI: 10.1053/j.gastro.2014.11.044. [ Links ]
81. Bortolotti M, Mari C, Lopilato C, et al. Sildenafil inhibits gastroduodenal motility. Aliment Pharmacol Ther 2001;15:157-61. DOI: 10.1046/j.1365-2036.2001.00917.x. [ Links ]
82. Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut 2002;50:758-64. DOI: 10.1136/gut.50.6.758. [ Links ]
83. Kuiken SD, Vergeer M, Heisterkamp SH, et al. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut 2002;51:212-8. DOI: 10.1136/gut.51.2.212. [ Links ]
84. Kuiken SD, Tytgat GN, Boeckxstaens GE. Role of endogenous nitric oxide in regulating antropyloroduodenal motility in humans. Am J Gastroenterol 2002;97:1661-7. DOI: 10.1016/S0002-9270(02)04180-1. [ Links ]
85. Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest 2000;106:803. DOI: 10.1172/JCI8273C1. [ Links ]
86. Vanormelingen C, Vanuytsel T, Masaoka T, et al. The normoglycaemic biobreeding rat: A spontaneous model for impaired gastric accommodation. Gut 2016;65:73-81. DOI: 10.1136/gutjnl-2014-308154. [ Links ]
87. Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011;140:1575-85. DOI: 10.1053/j.gastro.2011.01.046. [ Links ]
88. Sarnelli G, Sifrim D, Janssens J, et al. Influence of sildenafil on gastric sensorimotor function in humans. Am J Physiol Gastrointest Liver Physiol 2004;287:G988-92. DOI: 10.1152/ajpgi.00419.2003. [ Links ]
89. He CL, Burgart L, Wang L, et al. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology 2000;118:14-21. DOI: 10.1016/S0016-5085(00)70409-4. [ Links ]
90. He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001;121:427-34. DOI: 10.1053/gast.2001.26264. [ Links ]
91. Espin F, Rofes L, Ortega O, et al. Nitrergic neuro-muscular transmission is up-regulated in patients with diverticulosis. Neurogastroenterol Motil 2014;26:1458-68. DOI: 10.1111/nmo.12407. [ Links ]
92. Tjong YW, Ip SP, Lao L, et al. Role of neuronal nitric oxide synthase in colonic distension-induced hyperalgesia in distal colon of neonatal maternal separated male rats. Neurogastroenterol Motil 2011;23:666-e278. DOI: 10.1111/j.1365-2982.2011.01697.x. [ Links ]
93. García-González MA, Peña AS. Nitric oxide and inflammatory bowel disease. Rev Esp Enferm Dig 1998;90:870-6. [ Links ]
94. Perner A, Rask-Madsen J. Review article: The potential role of nitric oxide in chronic inflammatory bowel disorders. Aliment Pharmacol Ther 1999;13:135-44. DOI: 10.1046/j.1365-2036.1999.00453.x. [ Links ]
95. Martínez-Cutillas M, Mañé N, Gallego D, et al. EP2 and EP4 receptors mediate PGE2 induced relaxation in murine colonic circular muscle: Pharmacological characterization. Pharmacol Res 2014;90:76-86. DOI: 10.1016/j.phrs.2014.10.001. [ Links ]
96. Chaudhury A, He XD, Goyal RK. Role of myosin Va in purinergic vesicular neurotransmission in the gut. Am J Physiol Gastrointest Liver Physiol 2012;302:G598-607. DOI: 10.1152/ajpgi.00330.2011. [ Links ]
97. Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310-7. DOI: 10.1038/nature13085. [ Links ]
98. Jiménez M, De Diego M, Martínez-Cutillas M, et al. Purinergic and nitrergic inhibitory neuromuscular transmission in ganglionic, transitional and aganglionic segments from Hirschsprung's disease patients. Neurogastroenterol Motil 2015;27(S2):71Abs. [ Links ]
![]() Correspondence:
Correspondence:
Marcel Jiménez.
Departament of Cellular Biology, Physiology and Immunology.
Edificio V. Universitat Autònoma de Barcelona. 08193 Bellaterra.
Cerdanyola del Vallès, Barcelona. Spain
e-mail: marcel.jimenez@uab.es
Received: 20-10-2015
Accepted: 09-01-2016











 texto en
texto en