Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.11 Madrid nov. 2016
https://dx.doi.org/10.17235/reed.2016.3901/2015
CASE REPORTS
Endoscopic management of a gastric leak after laparoscopic sleeve gastrectomy using the over-the-scope-clip (Ovesco®) system
Manejo endoscópico de una fístula tras gastrectomía vertical mediante el sistema "over-the-scope-clip" (Ovesco®)
Yurena Caballero1, Eudaldo López-Tomassetti1, Ana Castellot2 and Juan Ramón Hernández1
Services of 1General and Gastrointestinal Surgery, and 2Gastroenterology. Complejo Hospitalario Universitario Insular-Materno Infantil de Las Palmas. Las Palmas de Gran Canaria, Spain
ABSTRACT
Laparoscopic sleeve gastrectomy is currently used for the management of morbid obesity. Gastric fistula is the primary life-threatening complication, and its resolution continues to be a strong challenge for surgeons. Multiple treatment options are available, ranging from conservative therapy to endoscopic use of clips or stents, and even surgical reoperation involving total gastrectomy or conversion to a different bariatric technique. The applicability of each individual option will depend on the type of fistula and the patient clinical status.
A clinical case is reported of a 29-year-old male patient with a body mass index at 49% who following laparoscopic sleeve gastrectomy had a delayed gastric fistula that failed to respond to conservative management but was successfully treated using the over-the-scope clip (Ovesco®) system.
Key words: Ovesco®. Gastric fistula. Obesity. Sleeve gastrectomy.
RESUMEN
La gastrectomía tubular laparoscópica se realiza hoy en día como procedimiento para el tratamiento de la obesidad. La fístula gástrica es la principal complicación que puede comprometer la vida del paciente y cuya resolución es un gran reto para el cirujano. Existen múltiples opciones de tratamiento que van desde un tratamiento conservador hasta medidas endoscópicas con clips o prótesis e incluso reintervenciones quirúrgicas que implican una gastrectomía total o la conversión a otra técnica bariátrica. La aplicabilidad de cada una de ellas va a depender del tipo de fístula y del estado general del paciente.
Se presenta un caso clínico de un paciente varón de 29 años de edad con índice de masa corporal de 49% que tras una gastrectomía vertical laparoscópica presenta una fístula gástrica tardía que fracasó al manejo conservador y cuya resolución se consiguió mediante el sistema "over-the-scope clip" (Ovesco®).
Palabras clave: Ovesco®. Fístula gástrica. Obesidad. Gastrectomía tubular.
Introduction
Laparoscopic sleeve gastrectomy was initially described by Gagner as a first stage for laparoscopic duodenal switch in patients with superobesity or high surgical risk. In these patients, after a significant weight loss, surgery was completed with a duodenal switch (1), or alternately a Roux-en-Y gastric bypass (2), after one year. However, a sleeve gastrectomy alone has proven effective in many of these patients, resulting in adequate weight loss in the mid run, and is currently performed by many authors as a standalone procedure given its technical simplicity.
Gastric fistula and staple line bleeding are the primary complications arising from this procedure (3,4). The management of staple line fistula is still controversial and no ideal therapy is available, existing in the literature multiple procedures described for its resolution. Management variability depends on time since surgery and patient status, and procedures described range from endoscopic interventions using clips or stents to repeat surgery that may entail a Roux-en-Y gastric bypass or even a total gastrectomy.
The over-the-scope clip (Ovesco®) system has been used for colonic perforation (5), and some current reports describe its use for fistula following sleeve gastrectomy (6). We report the case of a patient with staple line fistula after sleeve gastrectomy who was successfully treated using the over-the-scope clip (Ovesco®) system.
Case Report
A 29-year-old male patient with a body mass index at 49% (weight, 160 kg; height, 181 cm) and no comorbidities underwent laparoscopic sleeve gastrectomy calibrated with a 48F Foucher (1.6 cm in diameter). The staple line was reinforced using continuous 2/0 silk suture. On the second day after surgery methylene blue was administered with no evidence of staining in the drain output, and the patient was discharged the next day with liquid diet.
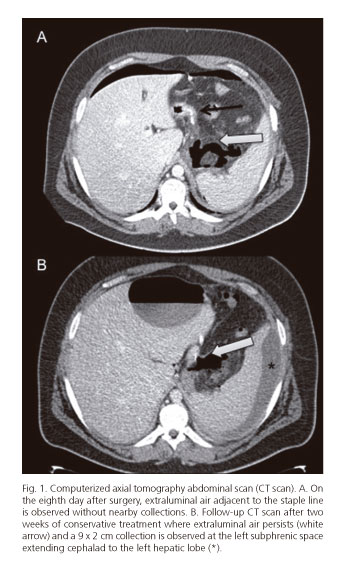
On the eighth day after surgery the patient presented to the emergency room with abdominal pain, no fever, and no associated increased heart rate. He reported no vomiting or other symptoms. Blood tests revealed leukocytosis with left shift, and a CT scan showed extraluminal air adjacent to the staple line with no nearby collections (Fig. 1A). Staple line fistula was suspected and conservative measures were selected including fasting, broad-spectrum antibiotics and parenteral nutrition.
Outcome was favorable. However, a follow-up CT scan after two weeks revealed a 9 x 2 cm collection at the left subphrenic space extending cephalad to the left hepatic lobe, and a percutaneous drain was placed (Fig. 1B). A gastrografin EGD study showed a good passage of contrast into the duodenum with no stenoses of leakage into the peritoneum.
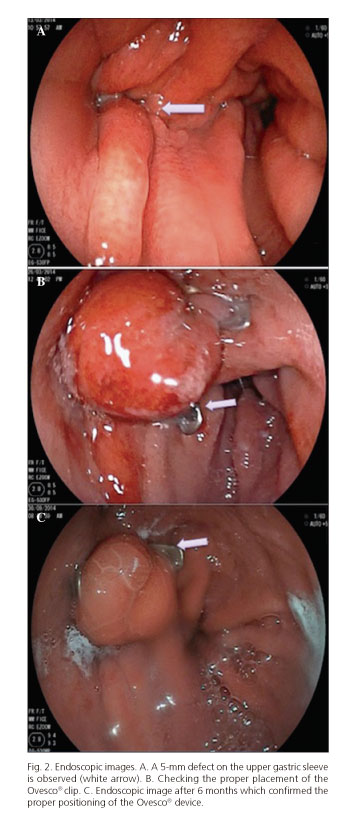
Despite conservative therapy and after 4 weeks on digestive rest, broad-spectrum antibiotics (Tienam® 500 mg every 8 hours; imipenem) and enteral nutrition with a nasojejunal tube, follow-up CT scans kept on showing a persistent fistula (extraluminal air bubbles adjacent to the staple line and fluid at the left subphrenic space), hence and upper GI endocospic procedure was suggested, which revealed a 5-mm defect on the upper gastric sleeve; an over-the-scope clip procedure was attempted for fistula closure (Fig. 2A).
The patient was placed in the supine position under sedation. A therapeutic endoscope (Fujinon 250) was used. The fistula was first localized and then its borders underwent electrocoagulation with argon to facilitate subsequent healing. A plastic cap fitted with a 12/6 clip was placed on the gastroscope's tip; near the lesion the involved borders were sucked into the cap. The clip was then released under the control of a manually operated wheel, which approximated, compressed and closed the perforation borders. Finally, proper clip placement was ascertained on endoscope withdrawal (Fig. 2B). On some occasions it is recommended that a water-soluble contrast can be instilled via the endoscope's working channel to ensure tightness following clip placement; however, this was not considered for our patient since proper placement was ascertained upon endoscope withdrawal and no leakage was identified.
A new endoscopic procedure was carried out after 24 hours, which acknowledged of a proper fistula closure. After this procedure the patient began to ingest liquids and was discharged four days after clip placement. He was then followed up on an outpatient basis and remained symptom-free. A repeat endoscopy was performed after 6 months confirming the proper positioning of the Ovesco® device and fistula closure (Fig. 2C). After one year the patient remains asymptomatic and his weight loss remains above 75% (current weight, 82 kg).
Discussion
Staple line fistula in sleeve gastrectomy is considered to be a life-threatening complication. Incidence ranges from 0.7% to 5% among the various reported series (3), a recent meta-analysis yielding 2.2% (6). This complication may manifest through the wound, in the drain or as an abdominal collection near the staple line (8). It is primarily located in the upper portion of the vertical gastrectomy adjacent to the esophagogastric junction, hence its challenging management.
Fistulas have been classified according to their timing or to imaging study findings. In the first case they are classified as early (within 3 days after surgery), intermediate (between 4 and 7 days postoperative), and late (after 8 days) (3,9). Rosenthal categorizes fistulas as acute (within 7 days after surgery), early (between 1 and 6 weeks), late (after 6 weeks), and chronic (after 12 weeks) (10).
Regarding imaging findings, fistulas may be type 1 or subclinical (local leakage without general dissemination or fistulous tract to the pleura or abdominal cavity, or contrast in drain material) or type 2 or clinical (widely spread intraabdominal leakage or spread to the pleura or abdominal cavity (3,8). The above patient had a late type-1 fistula. A different classification is based on CT findings according to collection size: type 1 (collection < 5 cm in the left hypochondrium), type 2 (collection > 5 cm), and type 3 (diffuse intraabdominal collections) (11).
No gold standard treatment is available; however, several alternatives have been posited. Management is decided according to the time of fistula diagnosis with respect to surgery, radiographic findings, and patient overall status. For early fistulas most authors consider exploratory laparoscopy as preferable for draining, washing and suturing (12). In contrast, surgical reoperation is deemed inappropriate for late type-1 fistulas as systemic inflammatory response may have induced dense inflammatory changes and fibrosis potentially complicating simple closure. Therefore, an initial conservative therapy based on fasting, parenteral nutrition, broad-spectrum antibiotics and percutaneous drainage of potential collections is usually selected. This sort of management, however, usually fails and other measures are necessary.
Several endoscopic treatments have been reported, including metallic or plastic self-expandable stents, clips and biologic glues, with varying outcomes (13). The primary limitations of stents in closing digestive perforations or fistulas are their high migration rate and their potentially challenging withdrawal. Standard clips only span perforations equal to or smaller than 1 cm (14). In the series reported by Moon, among 15 cases of gastric fistula 5 were managed with conventional clips or fibrin glue, and only one required repeat surgery (12). In 6 conservative treatment failures stents were successful for 50% initially, and fistulas were ultimately closed with a second stent in two additional patients; only one patient required surgery for persistent fistulas. Rosenthal suggested that gastric fistula closure with a stent is unlikely for fistulas of over 30 days standing (10).
For late fistulas failing to respond to conservative therapy, endoscopic closure with the over-the-scope clip (Ovesco®) device is an option. Initially described for colonic perforations, its use was extended to GI bleeding and gastrointestinal perforation. Its benefit derives from its circumferential beartrap-like sealing that involves the muscular layer or even the whole thickness of the gastric wall by applying a constant force with minimal stress on surrounding tissues. This system represents a new kind of endoscopic clip with increased strength and improved tissue capture as compared to conventional clips. It is made with nitinol, a superelastic biocompatible metal that may be used as long-term implant. The main technical difficulties of this procedure stem from the reduced caliber of the gastric sleeve, which hinders distal maneuvers with the endoscope, and the usual anatomical site of fistulas immediately past the cardia. Furthermore, a retroflexed endoscope is on occasion required, and successful clip placement becomes laborious.
Few series describe its use in patients after sleeve gastrectomy. Mercky et al. report their experience in 30 patients treated with the Ovesco® device for digestive fistulas, including 18 fistulas following sleeve gastrectomy; an initial clip was effective for 11 cases, 4 patients required additional procedures, and only two failures occurred (14). The authors conclude that Ovesco® clips for fistulas secondary to sleeve gastrectomy were more effective as compared to other causes of GI fistula. Surace et al. also reported a heterogeneous group GI fistula cases managed with Ovesco®, their reported success rate was 91% for fistulas secondary to vertical gastrectomy (15). The team led by Keren reported another series of 26 patients with fistulas secondary to sleeve gastrectomy where the Ovesco® device was used; of these, 21 were successful (80.76%) with a mean period of 20 days to full oral diet reintroduction (16).
In the above case a late fistula could be solved using the Ovesco® clip device, avoiding a reoperation. The related literature is sparse but the technique should be taken into account, given its results and simplicity, as an alternative for fistula closure after sleeve gastrectomy, particularly in the case of delayed fistulas, before making a decision for repeat surgery, as it may repair a gastric fistula while minimizing morbidity and mortality.
Chronic fistula represents a challenge for surgeons given the scarce possibilities available regarding closure using minimally invasive procedures. In cases where the previously described treatments fail, a new surgery should be considered. Potential surgical strategies that may be carried out range from total gastrectomy (17), which is considered as highly aggressive for a benign condition such as obesity, to conversion to gastric bypass. Chouillard describes another surgical option for chronic fistula, which consists of a Roux-en-Y fistula-jejunostomy (12).
Given the variability of fistula management a consensus should be reached on an ideal therapy for each fistula type. Moon et al. suggest the following algorithm (12): a) for smaller fistulas < 1 cm endoscopic closure with clips or biologic glue may be attempted; b) if these procedures fail or for larger fistulas stent placement may be attempted; and c) for persistent fistulas despite prior measures or for chronic fistulas a reoperation should be considered for a potential conversion to gastric bypass.
To conclude, gastric fistula after laparoscopic sleeve gastrectomy must be approached by a multidisciplinary team involving bariatric surgeons and endoscopists to suggest therapy options on an individual basis. Fistula timing and patient status are the primary determinants of treatment, and less invasive procedures should always be attempted first in order to prevent the complications associated with repeat surgery. For any gastric fistula not manifesting within the first few days after surgery Ovesco® offers a promising alternative with potential for successful closure and minimal complications.
References
1. Gagner M, Chu CA, Quinn T. Two-stage laparoscopic biliopancreatic diversion with duodenal switch: An alternative approach to super-super morbid obesity. Surg Endosc 2003;16:S069. DOI: 10.1381/096089203322190907. [ Links ]
2. Regan JP1, Inabnet WB, Gagner M, et al. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 2003;13:861-4. DOI: 10.1381/096089203322618669. [ Links ]
3. Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg 2009;19:1672-7. DOI: 10.1007/s11695-009-9884-9. [ Links ]
4. Deitel M, Gagner M, Erickson AL, et al. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis 2011;7:749-59. DOI: 10.1016/j.soard.2011.07.017. [ Links ]
5. Díez-Redondo P, Blanco JI, Lorenzo-Pelayo S, et al. A novel system for endoscopic closure of iatrogenic colon perforations using the Ovesco® clip and omental patch. Rev Esp Enferm Dig 2012;104:550-2. DOI: 10.4321/S1130-01082012001000009. [ Links ]
6. Aly A, Lim HK. The use of over-the-scope clip (OTSC) device for sleeve gastrectomy leak. J Gastrointest Surg 2013;17:606-8. DOI: 10.1007/s11605-012-2062-8. [ Links ]
7. Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: A systematic review and meta-analysis of 9,991 cases. Ann Surg 2013;257:231-7. DOI: 10.1097/SLA.0b013e31826cc714. [ Links ]
8. Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. DOI: 10.1046/j.0007-1323.2001.01829.x. [ Links ]
9. Csendes A, Burdiles P, Burgos AM, et al. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg 2005;15:1252-6. DOI: 10.1381/096089205774512410. [ Links ]
10. Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Díaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: Best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis 2012;8:8-19. DOI: 10.1016/j.soard.2011.10.019. [ Links ]
11. Nedelcu M, Skalli M, Delhom E, et al. New CT scan classification of leak after sleeve gastrectomy. Obes Surg 2013;23:1341-3. DOI: 10.1007/s11695-013-1002-3. [ Links ]
12. Moon RC, Shah N, Teixeira AF, et al. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis 2014;11:54-9. DOI: 10.1016/j.soard.2014.07.005. [ Links ]
13. Puig CA, Waked TM, Baron TH Sr, et al. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis 2014. DOI: 10.1016/j.soard.2013.12.018. [ Links ]
14. Mercky P, González JM, Aimore Bonin E, et al. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc 2015;27:18-24. DOI: 10.1111/den.12295. [ Links ]
15. Surace M, Mercky P, Demarquay JF, et al. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc 2011;74:1416-9. DOI: 10.1016/j.gie.2011.08.011. [ Links ]
16. Keren D, Eyal O, Sroka G, et al. Over-the-scope clip (OTSC) system for sleeve gastrectomy leaks. Obes Surg 2014;16. DOI: 10.1007/s11695-014-1540-3. [ Links ]
17. Ben Yaacov A, Sadot E, Ben David M, et al. Laparoscopic total gastrectomy with Roux-Y esophagojejunostomy for chronic gastric fistula after laparoscopic sleeve gastrectomy. Obes Surg 2014;24:425-9. DOI: 10.1007/s11695-013-1162-1. [ Links ]
![]() Correspondence:
Correspondence:
Yurena Caballero Díaz.
Service of General and Gastrointestinal Surgery.
Complejo Hospitalario Universitario Insular-Materno Infantil de Las Palmas.
Av. Marítima del Sur, s/n.
35016 Las Palmas de Gran Canaria, Spain
e-mail: yure_hop@hotmail.com
Received: 30-07-2015
Accepted: 18-08-2015











 texto en
texto en