Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.8 Madrid ago. 2018
https://dx.doi.org/10.17235/reed.2018.5435/2017
ORIGINAL PAPERS
A retrospective analysis of patients with gallbladder cancer: surgical treatment and survival according to tumor stage
1Unidad de Cirugía Hepato-Bilio-Pancreática y Trasplante de Órganos Abdominales. Hospital Universitario 12 de Octubre. Departamento de Cirugía. Facultad de Medicina. Universidad Complutense de Madrid. Madrid, Spain
INTRODUCTION
Gallbladder cancer (GBC) is the most common biliary neoplasm and the sixth most common tumor in the digestive system 1,2. Estimations suggest that 11,740 patients were diagnosed with gallbladder and bile duct cancer in the United States in 2017, which resulted in 3,830 deaths from GBC 1,3. The incidence varies according to geographic regions and ethnic/racial groups. The highest rates are reported in Chile, northeastern Europe, Israel, American Indians and Mexican Americans 2,4.
GBC has a poor prognosis, with a mean survival of six months and a 5-year survival rate of 5%. Risk factors for gallbladder cancer include cholelithiasis, obesity, gallbladder polyps > 1 cm, chronic gallbladder inflammation, environmental exposure and genetic alterations 4.
GBC is usually diagnosed taking into account the presence of a growth associated with regional adenopathies and distant metastases. However, 47% of these tumors are incidentally diagnosed following the examination of a cholecystectomy specimen 5. The incidence of gallbladder cancer in cholecystectomy specimens ranges from 0.2% to 3% 6,7,8,9.
Only a complete tumor resection (R0) offers a possibility of cure. However, the criteria for surgical resection are highly variable according to tumor stage. Surgical techniques range from simple cholecystectomy to right-side hepatectomy. Therefore, surgery adjusted to tumor stage is the only therapy that has somewhat improved the mean survival to date. However, the 5-year survival rates are still very low.
The primary goal of the study was to analyze and describe the epidemiological, clinical and therapeutic characteristics of patients diagnosed with GBC. The secondary endpoint was to assess survival according to tumor stage and surgical treatment.
MATERIAL AND METHODS
A descriptive, retrospective analysis of patients with a pathological diagnosis of GBC from January 2000 to January 2012 was performed. A total of 92 cases of GBC were collected during this period. The medical records were reviewed of patients with histologically confirmed GBC that were recorded in the tumor registry. Demographic, clinical, laboratory, radiological and histological variables were collected. Furthermore, data regarding surgical procedure, chemotherapy and survival according to tumor stage were also collected. Staging was performed according to the American Joint Committee on Cancer (AJCC) TNM Classification, 7th edition 10.
Cases of suspected GBC (radiographic finding) or incidental GBC at histology were presented to a multidisciplinary committee (gastroenterologists, oncologists, radiologists and surgeons). The indications for surgery and adjuvant therapy were discussed for each patient. Similarly, the follow-up of patients that underwent an R0 resection was performed according to the hospital protocol. Regular controls were performed by oncologists and surgeons every three months during the first two years and every six months thereafter over five years.
The initial surgery was based on simple or extended cholecystectomy which included radical cholecystectomy (cholecystectomy plus 2-3-cm resection of the surrounding liver parenchyma and hepatic hilar lymphadenectomy), cholecystectomy plus hepatectomy (bisegmentectomy IVb-V) and hepatic hilar lymphadenectomy. A potentially curative surgery was indicated when imaging tests or prior surgery suggested the feasibility of a tumor-free resection (R0). Similarly, palliative surgery was performed to relieve GBC-related symptoms or to prevent complications when a complete resection was not possible, for instance, in cases of perforated cholecystitis, bile duct obstruction, etc.
With regard to patients with residual tumor after examination of the surgical specimen, rescue surgery was considered as follows: in stage T1a (tumor invasion of the lamina propria), simple cholecystectomy was envisaged as a curative treatment with no need for rescue surgery; in stage T1b (tumor invasion of muscle layer) and T2 (perimuscular invasion without extension beyond the serosa or to the liver), rescue surgery consisted of a radical cholecystectomy. Bisegmentectomy IVb-V was considered for patients with liver invasion by contiguity, whereas a right hepatectomy was performed in patients with extensive liver invasion and/or invasion of the right sided vessels or bile ducts with an R0 intent.
Disease-free survival was defined as the interval between GBC surgery and radiographic or histological relapse. Survival analysis was censored in December 2017 and all patients had at least one year of follow-up.
Statistical analysis
Absolute frequencies and relative frequencies were used to describe qualitative variables as percentages. Quantitative variables were expressed as a mean and standard deviation when the distribution was normal and as the median 25th and 75th percentile when the distribution was not normal. Quantitative variable normality was assessed with the Kolmogorov-Smirnov test.
The Chi-squared test or Fisher's exact test was used to assess relationships between qualitative variables. Quantitative variable behavior was assessed for each independent variable using the Student's t-test. Nonparametric tests were used when normality could not be assumed.
Patient survival was estimated using the actuarial or Kaplan-Meier method and survival rates were compared using the log-rank test. A p value < 0.05 was considered as statistically significant. Study data were collected and analyzed using the SPSS 17.0 software.
RESULTS
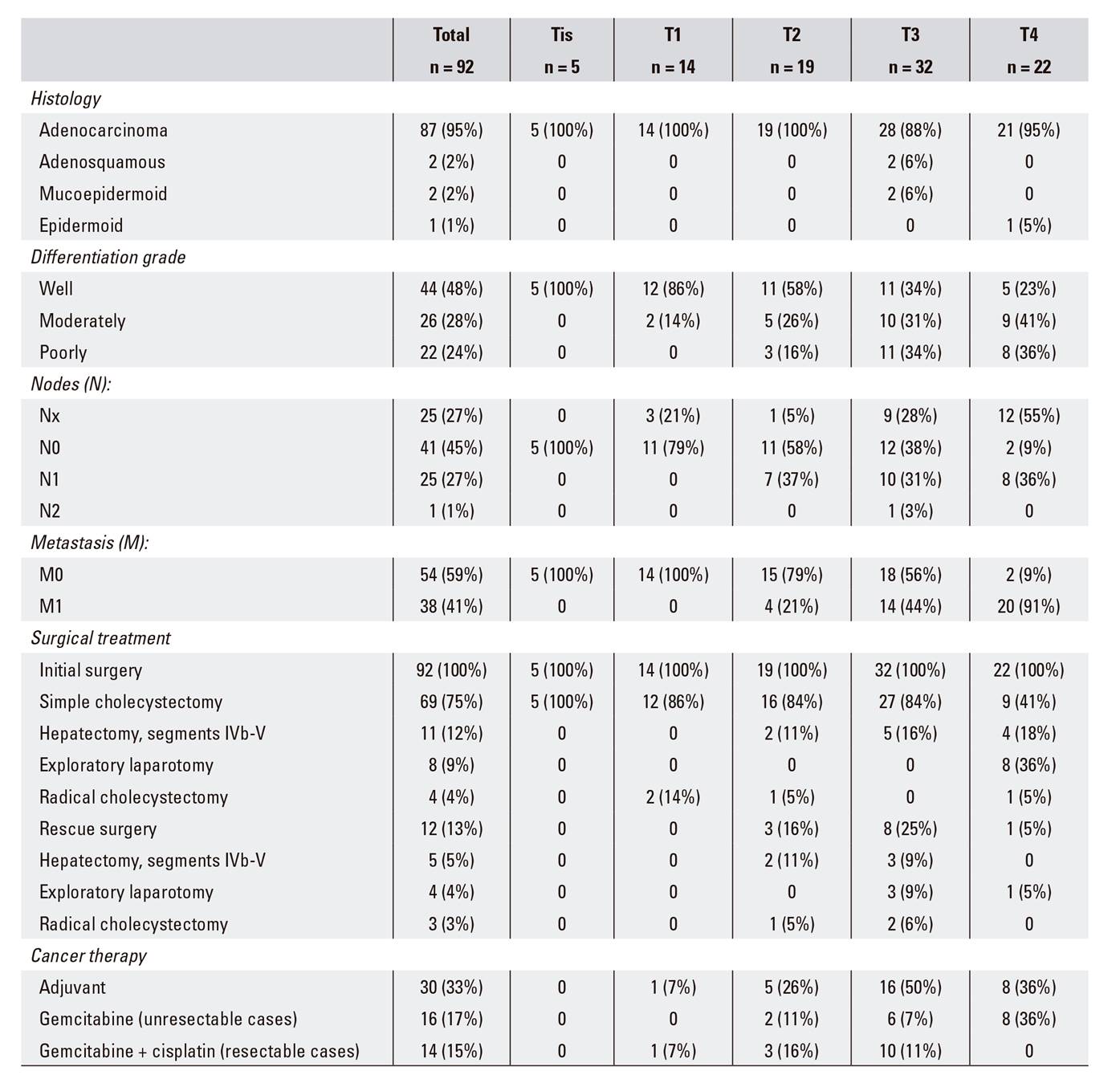
The mean age of cases was 72 ± 11 years, 64 (70%) patients were female and 28 (30%) patients were male. Relevant histories included blood hypertension (62%), diabetes (33%), smoking (22%) and drinking (11%). The main symptoms included abdominal pain (78%), nausea (58%), anorexia (55%) and jaundice (45%). Relevant laboratory data included a shift in liver function towards a predominantly cholestatic pattern. Furthermore, 40 (43%) patients had increased CEA levels and 53 (58%) had abnormal CA19.9 levels with a median of 52 U/ml (Table 1).
Table 1 Demographic, clinical, laboratory and radiographic characteristics of patients diagnosed with gallbladder cancer
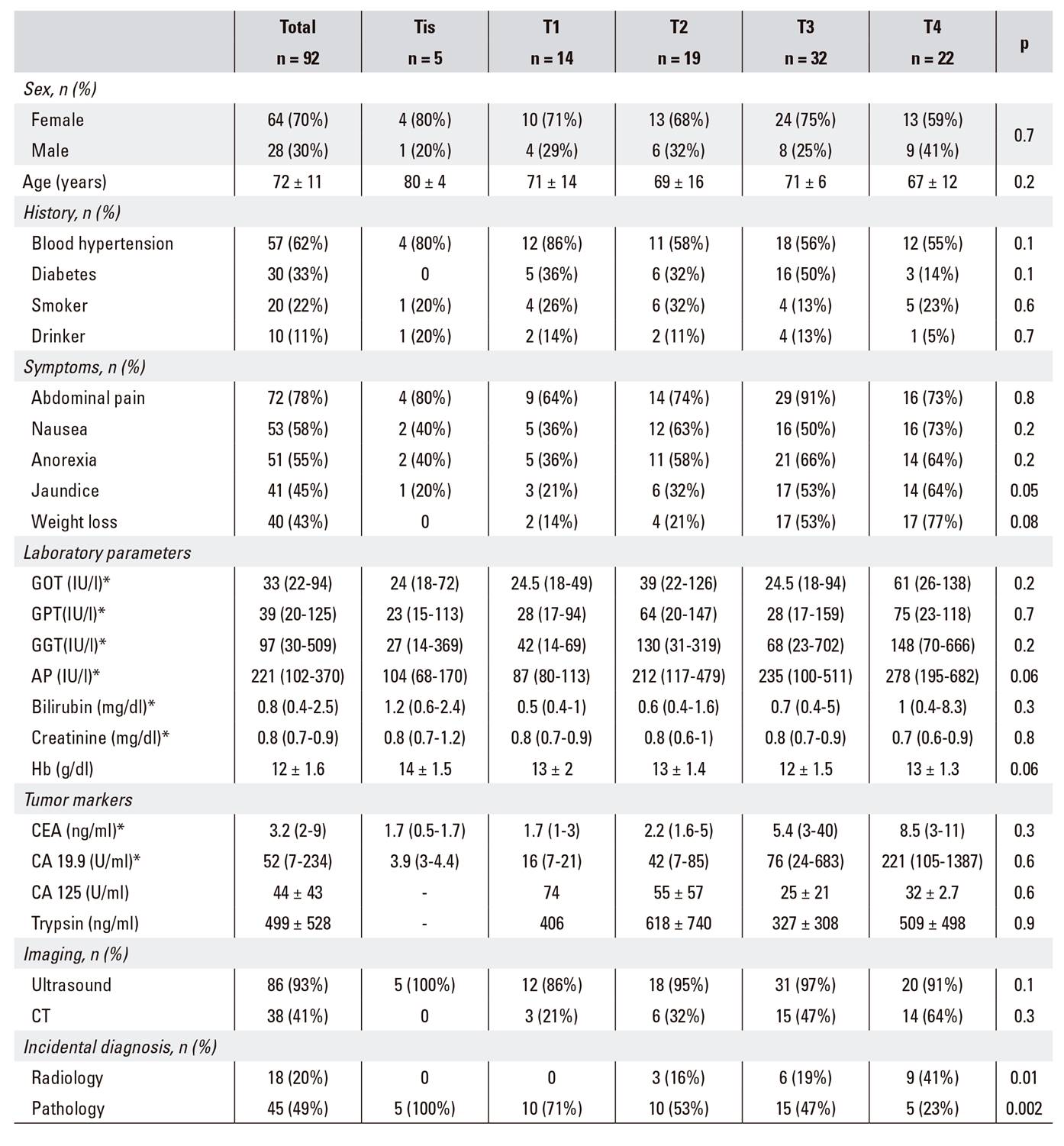
*Non-normal distribution variable, expressed as median (p25-p75).
All patients underwent imaging tests, mainly abdominal ultrasound (93%) followed by abdominal computed tomography (CT) (41%). Imaging studies revealed cholelithiasis in 89 (97%) patients, intra- and/or extrahepatic bile duct dilation in 40 (43%) patients, cholecystitis in 40 (43%) patients and vesicular polyps in nine (10%) patients.
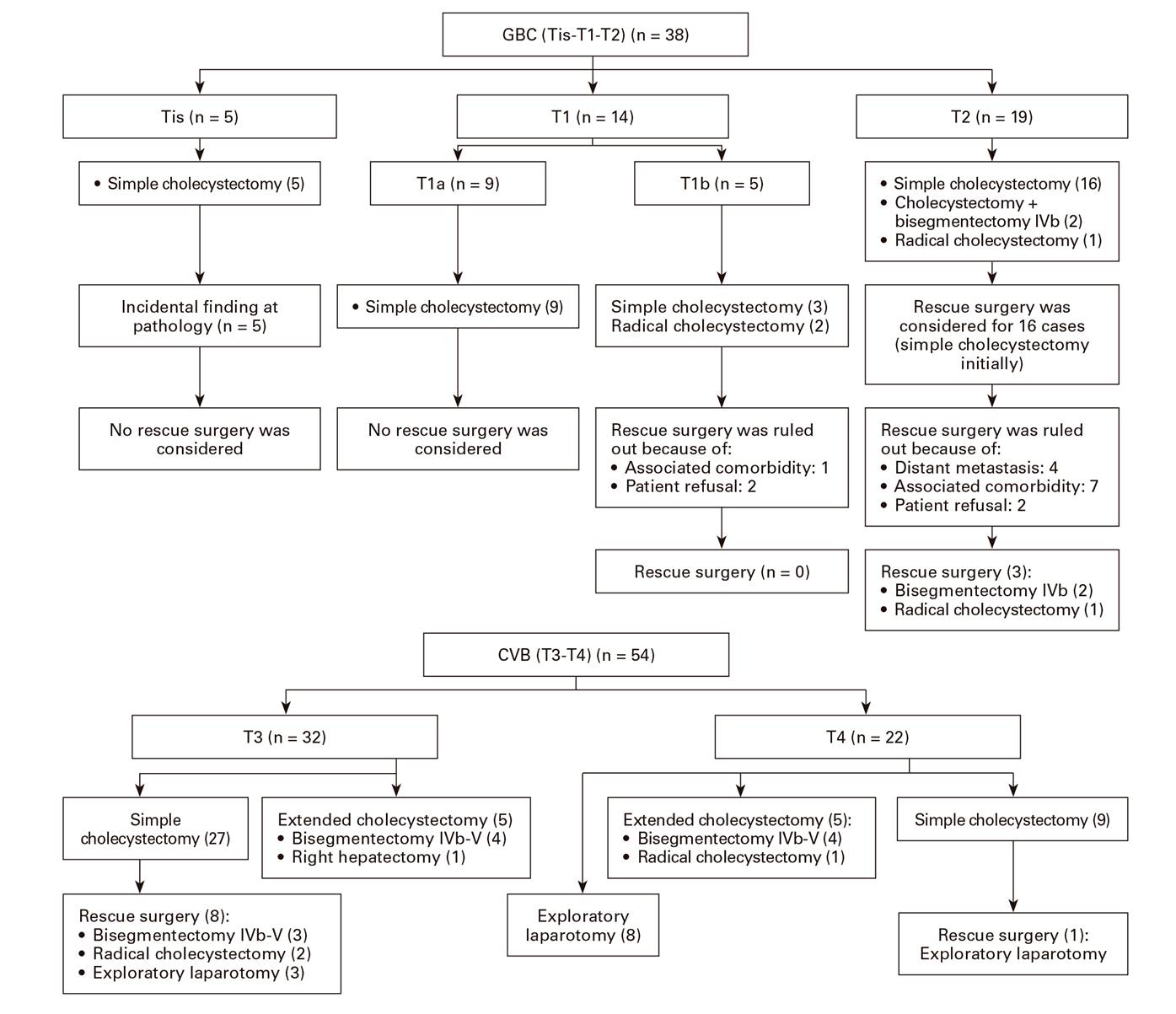
Surgery was performed in all 92 patients; the primary indication was most frequently acute cholecystitis (43%) followed by cholelithiasis (37%) and a preoperative diagnosis of GBC (20%). Laparoscopy was used initially in 21 (23%) patients and led to a subsequent laparotomy in 12 cases. Surgical procedures performed as an initial surgery included (Fig. 1) simple cholecystectomy in 69 (75%) patients and cholecystectomy plus hepatectomy in eleven (12%) patients; the latter consisted of a bisegmentectomy IVb-V in ten cases and extended right hepatectomy in one case. Furthermore, eight patients underwent an exploratory laparotomy that revealed advanced disease. All cases underwent palliative surgery due to perforated acute cholecystitis 3 and bile duct obstruction 5. The pathological diagnosis was adenocarcinoma in 87 (95%) patients, adenosquamous carcinoma in two (2%), mucoepidermoid carcinoma in two (2%) and squamous-cell carcinoma in one (1%) patient.
Rescue surgery was performed in 12 (13%) patients and eight patients had a curative resection (R0) (radical cholecystectomy in three and bisegmentectomy IVb-V in five). The remaining patients (four) had unresectable metastatic disease (Table 2).
With regard to the T parameter of the TNM system, five patients were Tis, 14 were T1, 19 were T2, 32 were T3 and 22 were T4. With regard to the N parameter, 26 (28%) patients had node tumor involvement; the histology was negative in 41 (45%) cases and not assessable (Nx) in 25 (27%) cases. Furthermore, metastases (M1) were seen in 38 (41%) patients.
Infiltration by contiguity was seen at the omentum (seven patients), duodenum (seven) and colon (two). In addition, eight (9%) patients had synchronous tumors (two prostate, two colonic, one gastric, one renal, one meningioma, and one skin) and ten (11%) had metachronous tumors (three gastric, three colorectal, two breast, one ovarian, one prostate).
Adjuvant therapy was used in 30 (33%) patients; gemcitabine was administered in 16 subjects with unresectable tumors and gemcitabine plus cisplatin, in 14 subjects with previously resected tumors (Table 2).
Postoperative mortality (within 30 days) was 10% (nine patients). Four patients died due to metastatic disease and these cases had only undergone exploratory laparotomy as an initial surgery. Three patients died from biliary or hepatic sepsis; two patients, following simple cholecystectomy; one patient, after extended cholecystectomy). One patient died from liver failure after rescue surgery consisting of a right hepatectomy and one patient died from pneumonia after a simple cholecystectomy.
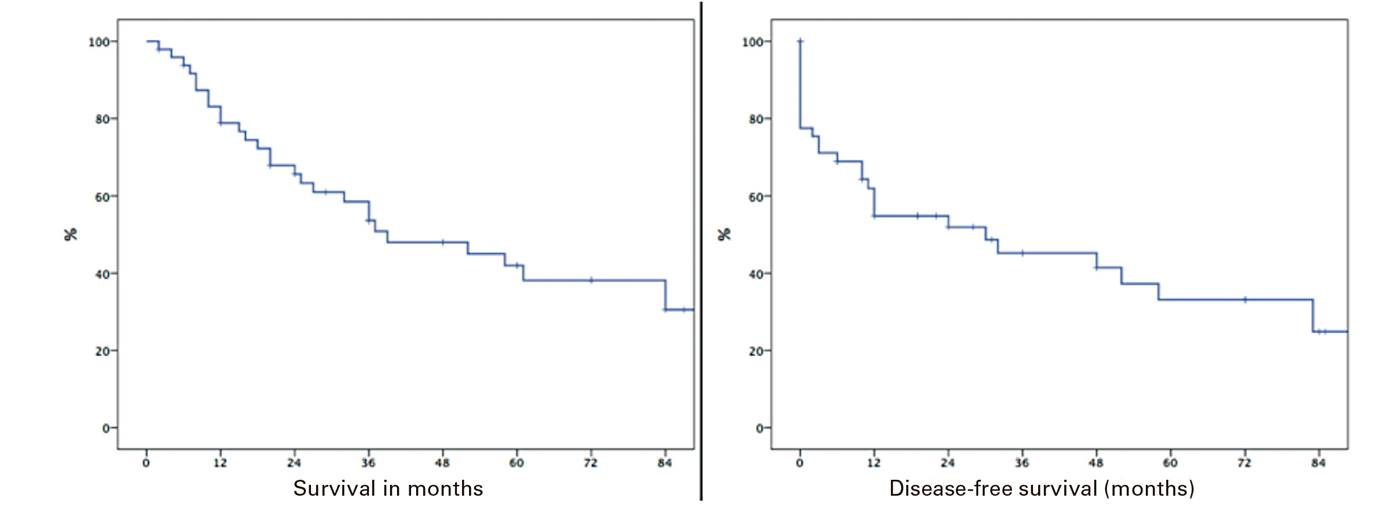
The median survival in our series was 12.5 months (0-96) and the survival rate after R0 surgery was 78%, 58.5% and 42% at one, three and five years, respectively. Furthermore, disease-free survival was 77.6%, 45.2% and 33.1% at one, three and five years, respectively (Fig. 2).
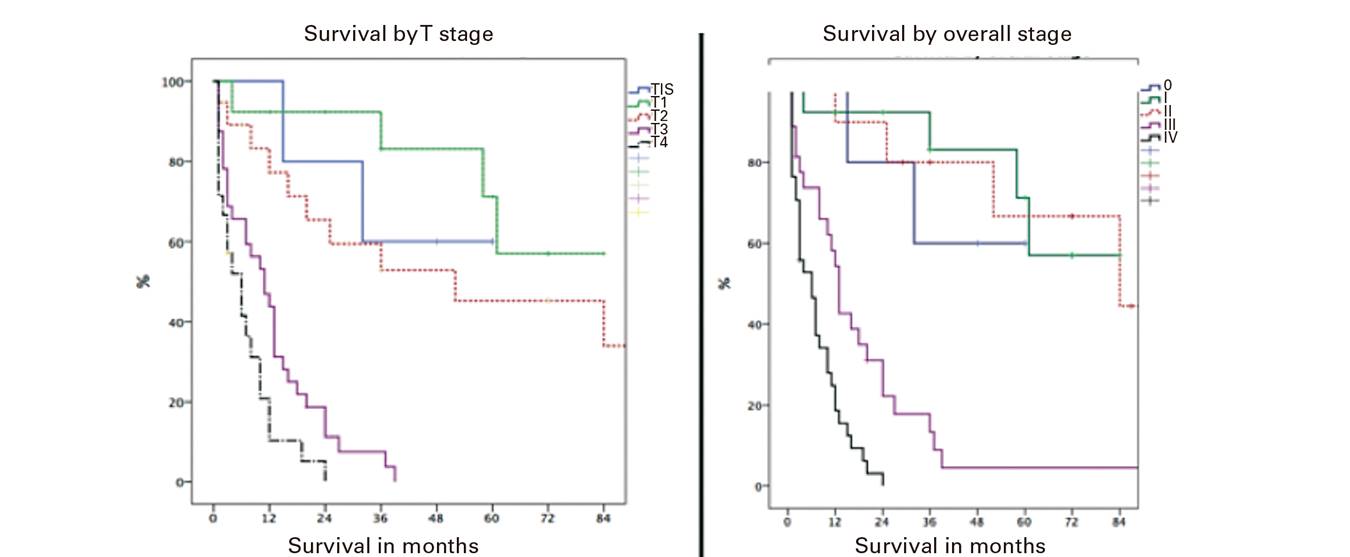
When analyzed according to TNM stage, survival was longer for patients with Tis-T1-T2 rather than T3-T4 (p ≤ 0.01) tumors. In addition, patients with stages 0, I and II survived longer than those with stages III and IV (Fig. 3).
DISCUSSION
Gallbladder cancer is a relatively rare condition in our setting, with a high mortality rate primarily due to the delayed diagnosis and the presence of tumor spread 11,12. Presentation symptoms are usually nonspecific and differentiating these tumors from other conditions with a higher prevalence, including biliary colic and chronic cholecystitis, is challenging. This complicates the preoperative review of the suspicious nature of the lesion and delays diagnosis. Abdominal pain is the most common symptom. Other symptoms that usually develop include jaundice and constitutional symptoms, both indicative of advanced tumor disease 13,14. In our study, abdominal pain was the most common symptom, reported by 78% of patients.
Serum carcinoembryonic antigen (CEA) and CA19.9 levels have been studied as screening markers, although they are nonspecific. CEA levels above 4 ng/ml have a specificity of 93% for the diagnosis of GBC compared to values in control subjects undergoing cholecystectomy for a benign biliary condition. However, the sensitivity is only 50% 15. Furthermore, elevated CA125, CA19.9, and CA242 levels have been reported in cases with distant lymph node metastasis 16. In our series, CA19.9 was the tumor marker that was most frequently elevated in GBC patients. With regard to the diagnostic approach used in our series, ultrasound was the most easily accessible and common initial test even though its yield rate for the initial diagnosis is not the highest among the various options available. The best imaging technique for GBC staging is contrast-enhanced CT, which may be supplemented by magnetic resonance (MR) cholangiography when biliary involvement is suspected 17.
A relevant aspect of this condition is the advanced stage of the tumor at diagnosis. Thus, a low percentage of patients are eligible for curative intent surgery; the latter is estimated to be only 25% of patients and only 16% of these will be alive after five years 18. In our series, complete tumor resection was only achieved in 55% of patients. Furthermore, disease-free survival at five years was 33%, a rate similar to that reported in other series 19.
The surgical management of GBC has evolved over the last few decades, and extended resections have been introduced into clinical management that have improved patient survival. Thus, extended or radical surgery without residual tumor (R0) may be the treatment of choice. However, randomized and controlled studies to define the optimum management have been sparse due to the low incidence of this tumor.
According to the latest guidelines, GBC management is based on TNM staging; simple cholecystectomy is considered to be the treatment of choice for T1a (confined to the mucosa) tumors. With regard to the surgical approach (laparoscopic vs laparotomic), recent studies suggest that the laparoscopic route is equivalent to the classic approach, even providing better results 19. Similarly, extended cholecystectomy is not appropriate in this stage, nor does it increase survival. However, when the tumor has invaded the cystic duct resection margin, resection of the biliary tract should be considered. Furthermore, no reported studies recommend the use of lymphadenectomy for stage T1a. In our series, simple cholecystectomy was considered as the treatment of choice for stage T1a and regional lymphadenectomy was deemed unnecessary. There is a lot of debate with regard to the management of patients with stage T1b (muscle layer infiltration) and T2 (serosal invasion, not beyond) cancer. The current tendency in surgery is radical cholecystectomy (extending to 2-3 cm away from the liver bed) or segment IVb-V resection. Both are associated with regional lymphadenectomy 17. However, the benefit of these surgical procedures is the subject of much debate since they are associated with low survival and high morbidity-mortality rates, and often no evidence of disease is collected 20.
Extended liver bed resection, either alone or associated with bisegmentectomy IVb/V for T2 tumors, may be a surgical approach based on tumor location 21. The gallbladder has a peritoneal (inferior) aspect and a hepatic (superior) aspect, with anatomic differences between both. There is no serosa on the hepatic aspect as the bladder is attached to the liver by connective tissue, which may facilitate tumor invasion by contiguity. Therefore, surgical management may vary depending on tumor location 22,23. Survival is not affected by liver resection in patients with T2 tumors on the peritoneal side, which represents a relevant prognostic factor. However, recurrence rates are higher when the surgery is not associated with a liver resection in T2 tumors on the hepatic side. In our series, rescue surgery was considered for 16 patients with stage T2 tumors (after finding GBC in the histology study following simple cholecystectomy). Of these, surgery was not performed in 13 cases due to distant metastasis, significant comorbidity or patient refusal (Fig. 1). Therefore, one patient underwent a radical cholecystectomy and two patients underwent a bisegmentectomy IVb-V; the surgery was curative and residual disease was found in pathological specimens.
Most patients in our study had stage T3/T4 (59%) tumors and were diagnosed after simple cholecystectomy. Rescue surgery was subsequently performed. As recommended, patients with stage T3 tumors should undergo rescue surgery, except for cases with a relevant comorbidity or advanced disease. Furthermore, patients with T3/T4 and lymph node involvement (N+) should be candidates for neoadjuvant therapy 17.
Lymph node metastasis is a prognostic factor and incidence by stage has been reported as follows: 0-2.5% in T1a, 5-16% in T1b, 9-30% in T2, 39-72% in T3, and 67-80% in T4. However, there is no consensus regarding the extent of the lymphadenectomy in association with radical cholecystectomy. Its relevance for survival and staging is recognized and allows to differentiate standard lymphadenectomy (confined to the hepatoduodenal ligament) from extended lymphadenectomy (celiac trunk, interaorto-caval space, etc.), with improved survival in the latter 24,25. In our series, regional lymphadenectomy was performed in all patients undergoing an extended cholecystectomy. Furthermore, patients without lymph node involvement (N0) have higher survival rates (p < 0.05) compared to subjects with node involvement (N+); at least 4-5 lymph nodes should be analyzed in order to rule out nodal involvement 26.
With regard to biliary tract resection, the prognostic value remains unknown, except in cases where its indication is clear, such as tumor infiltration of the cystic duct or common bile duct with no distant metastases 27. There are few experimental studies available with regard to adjuvant chemotherapy after tumor resection. The phase-III ABC-02 study (cisplatin + gemcitabine vs cisplatin alone) randomized clinical trial has shown a benefit for advanced biliary or metastatic tumors 28. Adjuvant therapy resulted in improved survival, even in patients with stage T3/T4 disease, nodal involvement and R1 resection 29. Radiotherapy is also increasingly used as it significantly reduces local recurrence rates and increases short-term survival 30. In our series, 30 patients received adjuvant therapy which was associated with radiotherapy in five patients.
A major bias in our study is its retrospective nature, which limited data collection and analysis. However, we believe that this review may contribute to the management of patients with this condition. To conclude, gallbladder cancer is an uncommon condition that may be incidentally diagnosed during the pathological analysis after cholecystectomy. Surgical management with complete (R0) tumor resection should be considered for all patients, provided their clinical status allows it. Therefore, treatment should be individualized for each patient.
BIBLIOGRAFÍA
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7-30. DOI: 10.3322/caac.21387 [ Links ]
2. Hundal R, Schaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109 [ Links ]
3. American Cancer Society. Cancer Facts & Figures 2017. [ Links ]
4. Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am 2014;94:342-60. [ Links ]
5. Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-89. DOI: 10.1002/jso.21141 [ Links ]
6. Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC Study Group. World J Surg 2011;35:1887-97. DOI: 10.1007/s00268-011-1134-3 [ Links ]
7. Pitt SC, Jin LX, Hall BL, et al. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg 2014;260:128-33. [ Links ]
8. Choi KS, Choi SB, Park P, et al. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol 2015;21:1315-23. DOI: 10.3748/wjg.v21.i4.1315 [ Links ]
9. Vinuela E, Vega E, Yamashita S, et al. Incidental gallbladder cancer: residual cancer discovered at oncologic extended resection determines outcome. A report from high- and low-incidence countries. Ann Surg Oncol 2017;24:2334-43. DOI: 10.1245/s10434-017-5859-6 [ Links ]
10. Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer stating manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471-4. DOI: 10.1245/s10434-010-0985-4 [ Links ]
11. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. DOI: 10.1002/ijc.21683 [ Links ]
12. Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol 2015;21(43):12211-7. DOI: 10.3748/wjg.v21.i43.12211 [ Links ]
13. Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4(3):167-76. DOI: 10.1016/S1470-2045(03)01021-0 [ Links ]
14. Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg 2006;243:767-71. DOI: 10.1097/01.sla.0000219737.81943.4e [ Links ]
15. Ramírez CP, Suárez MA, Santoyo J, et al. Actualización del diagnóstico y el tratamiento del cáncer de vesícula biliar. Cir Esp 2002;71:102-11. DOI: 10.1016/S0009-739X(02)71940-4 [ Links ]
16. Wang YF, Feng FL, Zhao XH, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol 2014;20(14):4085-92. [ Links ]
17. Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford) 2015;17(8):681-90. DOI: 10.1111/hpb.12444 [ Links ]
18. Löhe F, Meimarakis G, Schauer C, et al. The time of diagnosis impacts surgical management but not the outcome of patients with gallbladder carcinoma. Eur J Med Res 2009;14(8):345-51. [ Links ]
19. Lee SE, Jang JY, LIM CS, et al. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol 2011;17(2):174-80. [ Links ]
20. Watson H, Dasari B, Wyatt J, et al. Does a second resection provide a survival benefit in patients diagnosed with incidental T1b/T2 gallbladder cancer following cholecystectomy? HBP (Oxford) 2017;19(2):104-7. [ Links ]
21. Lee H, Choi DW, Park JY, et al. Surgical strategy for T2 gallbladder cancer according to tumor location. Ann Surg Oncol 2015;22(8):2779-86. DOI: 10.1245/s10434-014-4300-7 [ Links ]
22. Shindoh J, De Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg 2015;261(4):733-9. DOI: 10.1097/SLA.0000000000000728 [ Links ]
23. Lee W, Jeong CY, Jang JY, et al. Do hepatic-sided tumors require more extensive resection than peritoneal-sided tumors in patients with T2 gallbladder cancer? Results of a retrospective multicenter study. Surgery 2017;162(3):515-24. [ Links ]
24. Liu GJ, Li XH, Chen YX, et al. Radical lymph node dissection and assessment: impact on gallbladder cancer prognosis. World J Gastroenterol 2013;19(31):5150-8. [ Links ]
25. Sternby Eliard M, Lundgren L, Cahlin C, et al. Surgical treatment for gallbladder cancer - A systematic literature review. Scand J Gastroenterol 2017;52(5):505-14. DOI: 10.1080/00365521.2017.1284895 [ Links ]
26. Jung W, Jang JY, Kang MJ, et al. Effects of surgical methods and tumor location on survival and recurrence patterns after curative resection in patients with T2 gallblader cancer. Gut Liver 2016;10(1):140-6. DOI: 10.5009/gnl15080 [ Links ]
27. Gani F, Buettner S, Margonis GA, et al. Assessing the impact of common bile duct resection in the surgical management of gallbladder cancer. J Surg Oncol 2016;114(2):176-80. DOI: 10.1002/jso.24283 [ Links ]
28. Kasumova GG, Tabatabaie O, Najarian RM, et al. Surgical management of gallbladder cancer: simple versus extended cholecystectomy and the role of adjuvant therapy. Ann Surg 2017;226(4):625-31. DOI: 10.1097/SLA.0000000000002385 [ Links ]
29. Kim Y, Amini N, Wilson A, et al. Impact of chemotherapy and external-beam radiation therapy on outcomes among patients with resected gallbladder cancer: a multi-institutional analysis. Ann Surg Oncol 2016;23(9):2998-3008. DOI: 10.1245/s10434-016-5262-8 [ Links ]
30. Wang J, Narang AK, Sugar EA, et al. Evaluation of adjuvant radiation therapy for resected gallbladder carcinoma: a multi-institutional experience. Ann Surg Oncol 2015;22(Suppl 3):S1100-6. DOI: 10.1245/s10434-015-4685-y [ Links ]
Received: December 26, 2017; Accepted: February 03, 2018











 texto en
texto en