Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de la Sociedad Española del Dolor
versión impresa ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.28 no.3 Madrid may./jun. 2021 Epub 27-Sep-2021
https://dx.doi.org/10.20986/resed.2021.3911/2021
ORIGINALS
Prescribing habits for neuropathic pain management in Spain: results of the survey of the neuropathic pain working group of the Spanish Pain Society
1aHospital Universitari de Bellvitge. Barcelona, España
1bCoordinador del Grupo de Trabajo de Dolor Neuropático de la Sociedad Española del Dolor. España
2Centro de Salud Puerta del Ángel. Madrid, España
3Vocal del Grupo de Trabajo de Dolor Neuropático de la Sociedad Española del Dolor hasta 2020. Sociedad Española del Dolor. España
4Hospital POVISA. Vigo, España
5Hospital de la Princesa. Madrid, España
6Hospital Universitario Virgen de las Nieves. Granada, España
INTRODUCTION
Neuropathic pain (NP) is pain that occurs as a direct result of an injury or disease affecting the somatosensory system 1. The prevalence of NP in the population varies between 6.9 and 10 %, depending on the tool used for its diagnosis 2,3,4. These patients frequently suffer from depression and have a poor quality of life, with significant disability and vulnerability 5.
The latest drugs with indication for NP in international guidelines came onto the market more than ten years ago. These guidelines recommend starting with monotherapy and place the combination therapy on the second step 6,7. However, a considerable number of patients do not achieve sufficient pain relief or improvement in their quality of life with available drugs 8. NP treatment is effective in less than 50 % of patients and is also associated with significant adverse drug effects 7,9. Although there is evidence that more than 45 % of individuals with NP take two or more pain drugs, in a review it was only possible to find 21 high-quality studies of different combinations of systemic and topical drugs 10. In 2017, in Denmark 11, recommendations for combination therapy in NP were developed.
A 2012 publication 12 sought to unify the definition of refractory neuropathic pain (RNP) for epidemiological studies. RNP would be the one in which at least four drugs with a duration of each treatment of at least three months have failed, or until adverse effects prevent dose increases and where pain intensity has been reduced by less than 30 %. or it persists at 5 or more on a 0-10-point scale and/or a poor quality of life persists.
In 2019, The group of Special Interest in NP of the Italian Society of Neurology developed a consensus on the definition of pharmacoresistant NP 13 "when the patient does not reach the 50 % reduction of pain or an improvement of at least 2 points in the Patient Global Impression of Change, having used all drug classes indicated as first, second, or third line in the most recent and widely agreed international guidelines, for at least 1 month after titration to the highest tolerable dose" This consensus will help identify patients susceptible to invasive treatments.
RNP and pharmacoresistance forces us to be creative and audacious in trying to alleviate these patients. So far, there is no evidence to allow us to reach a consensus to guide us in the use of off-label drugs for NP treatments or the evidence is insufficient. In this perspective, the NP Working Group (WG) of the Spanish Pain Society (SED, in Spanish: Sociedad Española del Dolor) designed a survey to know the approach of NP by drugs, interventional techniques and off-label use of treatments in our environment. This article discusses only the drug treatment part.
MATERIAL AND METHODS
Design
Descriptive study using a self-administered questionnaire. The survey was distributed by e-mail to all SED members in two waves during the months of September and October 2019, and an announcement was published on the back cover of the Revista de la Sociedad Española del Dolor (Journal of the Spanish Pain Society), Volume 26, Number 5 (September-October 2019). It was designed through the Formsite(r) survey company, Chicago, Illinois, USA, with dichotomous, multiple-choice variables, Likert scale-type responses with 4 options, and free-field responses. The survey was categorized into 4 sections: Demographic data, medication not indicated, polypharmacy, and off-label uses. At the start of the questionnaire, a selection question was asked regarding if the respondents were using or not off-label treatments. Only those who answered yes proceeded to the whole set of questions. This was divided into the following blocks: Antiepileptics, antidepressants, antipsychotics, anesthetics, anti-NMDA, Cannabinoids (CNB), naltrexone (NTX), topical treatments, botulinum toxin (BTA), polypharmacy and off-label treatments. Botulinum toxin was included in the topical treatment section.
Study population
As of September 2019, SED had 1356 members registered in the company's database. Of these, 1285 have an operational e-mail address and have provided their consent to receive the SED newsletter. Since the survey was open, it did not collect names or addresses that could be identified to avoid conflict with the data protection law. To avoid duplication of data, duplicate data were deleted, gender, years of work experience, and IP address of the user's origin were crossed with data of the survey date, operating system, and browser used. Incomplete duplicate results and those whose percentage of responses did not cover at least the medication and demography sections or their execution time was less than 100 seconds, were discarded.
Collected variables
Four blocks of questions were collected: Demographic, pharmacological treatment, polypharmacy and off-label. The following data were evaluated in the demographic variables: Age, gender, specialty, years of work experience and workplace.
In the drug treatment block the items were:
Antiepileptics: Oxcarbazepine (OXZ), eslicarbazepine (ECZ), lamotrigine (LMT), phenytoin (FNT), lacosamide (LMD), levetiracetam, perampanel and zonisamide.
Antidepressants: Venlafaxine (VLF), desvenlafaxine (DVF), and bupropion.
Antipsychotics: Quetiapine (QTP) and levomepromazine.
Anesthetics: Propofol (PPF), sevoflurane (SFN), and intravenous lidocaine (IV Lido).
Anti-NMDA: Ketamine (KTM) and amantadine.
Topical treatments: Lidocaine gel or transmucosal cream, lidocaine patch 5 % (Li5 %), capsaicin 8 % (CPS) in off-label use, and the mixture of amitriptyline plus ketamine as ointment.
A section on the prescription of CNB, NTX and BTA was also created.
Each asked about the frequency of use using a Likert scale of 4 options ("used frequently", "occasionally", "I do not use but I have used it", and "I do not use and I have not used it") and for the scientific reasoning followed for its prescription with another scale of 5 options ("information from courses and conferences", "protocols of my workplace", "publications", "own experience", and "extrapolation of basic articles or mechanisms of action"). This last question was multiple-choice (multiple answers could be marked).
In the block of polypharmacy and off-label treatment use in NP, the items were: Percentage of patients who need to use polypharmacy, percentage of patients who currently are under polypharmacy, at what time it is prescribed (in the first line, if they do not respond to a first line, if they does not respond to the first two lines) and the opinion on the evidence regarding the treatment of NP with polypharmacy. The complete questionnaire can be accessed in the supplementary material annexed to the journal's website.
Statistical analysis
A descriptive analysis of the results of the questionnaire is performed. Categorical variables are presented as absolute frequency and percentage (%). Continuous variables are represented by the mean and its standard deviation. Since it is a descriptive study, there is no statistical significance.
RESULTS
A total of 150 out of the 1356 members who had registered in the SED responded to the survey in the 4 weeks available to answer. After the incomplete responses of less than 100 seconds were deleted, 110 surveys remained, from which a single duplicate record was deleted. The target population was only those active physicians, who were 1085 out of 1285. Thus, the response rate was 13.82 %, being 10.05 % after the invalid ones were ruled out.
1.st block. Demographic data: 48.72 % of respondents were women, more than half of respondents were anesthesiologists (52.29 %). The distribution by years of professional experience was mainly divided between the ranges of 10 to 20 years (30.77 %) and 20 to 30 years (34.62 %) of post-residence professional experience (Table 1).
Table I. Sociodemographic data

FF: Family Physician. PMR: Physical Medicine and Rehabilitation. Work experience and response time represented as mean and standard deviation, in parentheses. The rest are absolute values and percentages.
2.nd block. Polypharmacy: For polypharmacy questions there were only 82 answers (54.6 % of participants). Of the responses received, 21 % of physicians begin treatment of NP with polypharmacy, 43 % when patients do not respond to a first line, and 18 % after two single refractory lines (Figure 1). 40 % of respondents believe that there is insufficient evidence for the use of polypharmacy and 53.7 % believe that the current evidence is insufficient (Figure 2).

Fig. 1. Use of polypharmacy. Number of responses about when do they use combination therapy to treat neuropathic pain.

Fig. 2. Evidence on polypharmacy. Number of responses to the question of how much evidence do they believe there is about the use of polypharmacy in neuropathic pain.
3.rd block. Drugs: 70 % of respondents treated up to 30 % of their patients with NP with off-label drugs. 23.3 % used off-label drugs in 40-60 % of patients with NP and 6.6 % used off-label treatments in 70-90 % of patients. None of the responders used them in 100 % of patients. The group analysis of drugs used off-label was as follows:
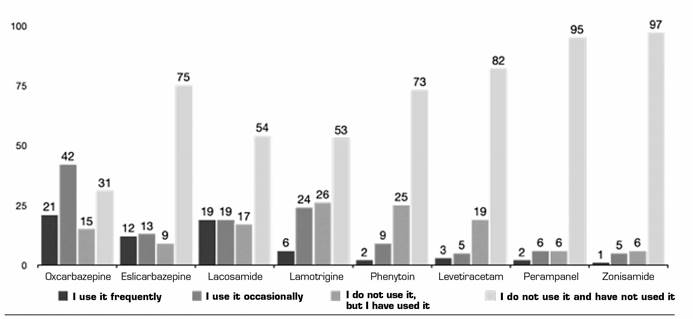
Anticonvulsants (ACV): The ACV most frequently used off-label was OXZ (19 % use it frequently), followed by LMD (17 % use it frequently) and ECZ (11 %). More than 80 % have not used other ACVs such as zonisamide or perampanel. For more details, please, see Figure 3. The majority of respondents report that the off-label use of ACV is based on information from publications (57.27 %), followed by information from courses and conferences in those using them frequently (20 %) and extrapolation from action mechanism (25.25 %) in those using them occasionally (Table 2).
Table II. Relationship of use of antiepileptics and origin of training and evidence

Courses and conferences: Training obtained from papers (oral presentations). Publications: Training or evidence obtained by the drug in clinic. Extrapolation: Training or evidence obtained by extrapolating basic science publications or mechanisms of action. Protocols in their workplace. Own experience with the drug: This question was of multiple choice. Each respondent could respond to several options.
Antidepressants (AD) and antipsychotics: VLF (28.5 % frequently and 43.1 % occasionally) and DVF (15.6 % frequently and 38.5 % occasionally) are the most frequently prescribed ADs for off-label use for pain. 4.5 % of respondents use QTP frequently and 24 % occasionally (Figure 4). Most respondents using AD or antipsychotics off-label report doing so based on publication results (53.15 %), followed by information obtained from courses and conferences (21.17 %) (Table 3).
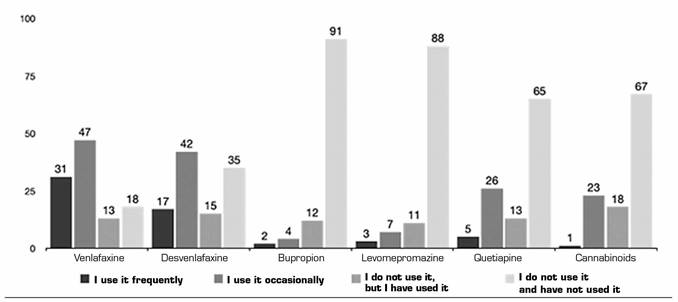
Fig. 4. The frequencies of use are represented in absolute numbers. The cannabinoids group is included, despite having its own section in the questionnaire, because of the psychotropic character of the tetrahydrocannabinol (THC).
Table III. Relationship of use of antidepressants/antipsychotics, and origin of training and evidence

Cannabinoid responses are included in this group. Courses and conferences: Training obtained by presentations. Publications: Training or evidence obtained by the drug in clinic. Extrapolation: Training or evidence obtained by extrapolating basic science publications or mechanisms of action. Protocols in their workplace. Own experience with the drug: This question was of multiple choice. Each respondent could respond to several options.
Anesthetics and others: In this group, LiIV and KMN were the most commonly used (36.7 % and 26.6 % frequently, and 25.6 % and 31.2 % occasionally) (Figure 5). In this group again the information received from publications is the main source (42.91 %). However, the second place is the extrapolation of basic science publications or mechanisms of action (18.39 %) and the third is information from courses and conferences (16.85 %) (Table 4).
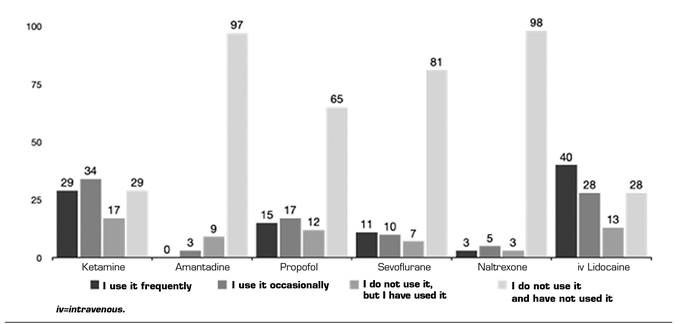
Fig. 5. The frequencies of use are represented as absolute numbers. Intravenous lidocaine is included, despite it belongs to another section of the questionnaire, to facilitate the understanding of the results.
Table IV. Relationship of use of anesthetics and source of training or evidence

Naltrexone responses are included in this group. Courses and conferences: Training obtained by presentations. Publications: Training or evidence obtained by the drug in clinic. Extrapolation: Training or evidence obtained by extrapolating basic science publications or mechanisms of action. Protocols in their workplace. Own experience with the drug: This question was of multiple choice. Each respondent could respond to several options
Topical drugs: The most frequently used topical treatments were Li5 % (off-label in postherpetic neuralgia [PHN]), with 53.2 % frequent use and 29.4 % occasional use, followed by CPS (face, genital area...) with 23.85 % frequent use and 29.4 % occasional use. Topical lidocaine in mucous membranes was also used relatively frequently (19 % frequently and 33 % occasionally). Master combinations such as amitriptyline and KTM were rarely used (Figure 6). The use of these treatments came mainly from publications (46.18 %), in this case followed by information obtained in courses and conferences (20.83 %), both for frequent and occasional use (Table 5).

Fig. 6. Frequency of off-label use of topical treatments. Lidocaine 5 % outside post-herpetic neuralgia. Capsaicin 8 % in other areas (face, genitals, mucous membranes or pediatrics, etc.). Amitriptyline+Ketamine mixture. BTA sc = Botulinum toxin type A subcutaneous.
Table V. Relationship of use of topical treatments and source of training or evidence

Botulinum toxin responses are included in this group. Courses and conferences: Training obtained by presentations. Publications: Training or evidence obtained by the drug in clinic. Extrapolation: Training or evidence obtained by extrapolating basic science publications or mechanisms of action. Protocols in their workplace. Own experience with the drug: This question was of multiple choice. Each respondent could respond to several options.
Other drugs: BTA was used very frequently by 44.9 % and occasionally by 33 %, compared with CNB that were not used in NP (67 %) (Figures 4 and 6). Again, the use of these treatments came mainly from publications (52.78 % for BTA and 62.5 % for CNB), in this case followed by information obtained in courses and conferences (19.44 % for BTA and 25 % for CNB) both for frequent or occasional use in BTA and occasional use in CNB.
DISCUSSION
According to our knowledge, this is the first time that an investigation has been performed on the approach to NP with the aim of knowing the need for polypharmacy and off-label drug use. Although it is only a descriptive study, it highlights the gap between clinical guidelines in NP, journal publications and reality in daily clinical practice. This confirms the difficulty of treating this type of pain 14. Thus, we believe that the information obtained, even having a relative weight, is of the utmost importance to be able to understand the approach of NP in our environment, since the respondents, for the most part, were specialists in the treatment of pain.
75 % of respondents said they used polypharmacy. Although there are some good quality studies showing superior efficacy of combinations of two drugs, the number of studies of combination therapies are scarce and with small sample sizes 10,14. Studies available to date do not support the recommendation of one specific drug combination over another 15, but do provide reasoning for combination therapy. For now, GBP combined with morphine or nortriptyline is the only combination that appears to be superior to monotherapy 7,16. Although the largest study (not placebo-controlled) showed no differences in efficacy or side effects between pregabalin (PGB) combined with duloxetine (DXT) 17. Therefore, the combination of PGB/GBP and Tricyclic Antidepressant (TCAD) DXT (level A for GBP-opioids or GBP-TCAD) can be considered as an alternative to increasing monotherapy doses for patients who do not respond to moderate-dose monotherapy 16. In addition, it should be noted that combinations can increase the risk of toxicity, so routine use requires careful risk-benefit assessment. To reduce this, a very common approach, sequential combination, is often used. This consists of starting monotherapy and continuing with "complementary" combination therapy in cases of partial response to treatment. According to the results obtained, this is what the majority of respondents seem to be doing. The combination of ≥ 2 analgesic agents in the treatment of NP is an attractive option because the combination therapy can improve analgesic efficacy 15 and has the potential to reduce the overall profile of side effects if synergistic effects allow dose reductions of combination drugs 18.
The answer to the question about the most frequent use of polypharmacy was mostly in patients who did not respond to a partial or total first line analgesic. According to the answers, this happens in 75 % of patients. This may be due to lack of efficacy at maximum doses of drugs or the association of different treatments looking for different targets, even though the maximum dose has not been reached with the first therapy; we have not explored this response and it is really difficult to know because clinical guidelines offer both possibilities 14,19. Despite the wide use of combination therapy, more than 92 % agree that there is not enough evidence about polypharmacy, which shows the need to endorse its use. This survey shows no relationship between the percentage of patients in polypharmacy and respondents' opinion of existing evidence. There are also no differences between the percentage of patients with combination of drugs with label use and with off-label use and the time of initiation. However, there is a difference between the percentage of patients in polypharmacy and the decision about when to prescribe it. When the percentage of patients in polypharmacy is more than 60 %, more prescription of initiation is found (more information can be seen in the figures in the annexes, as supplementary material, on the website).
As for the type of medication used, the most commonly used off-label antiepileptics are OXZ and LMD, followed by ECZ and LMT. However, others such as phenytoin and levetiracetam are used occasionally, or have been used relatively frequently previously. More than half of respondents claim to base their indication on specific publications, a percentage that amounts to more than 91 % if added to those that indicate that they are based on information collected from courses and conferences or that extrapolated from basic sciences. Only 6 % say they use their own experience for off-label use. However, for now, anticonvulsants in combination treatment have not shown a significant benefit in reducing overall pain 20. Only in chemotherapy-induced neuropathy, combination treatment with anticonvulsants or antidepressants has shown better efficacy. This again is another difference between what is seen in clinical guidelines or publications and clinical practice. It should be remembered that the long-term efficacy and safety of both in combination therapy remains unknown, as trials have been conducted with a short duration of time, without long-term follow-up.
Among antidepressants and antipsychotics, it is worth noting the use of VLF and DVF, with significant occasional and frequent use, compared with occasional or previously performed use of QTP and CNB. More than 50 % claim to base the information for indication on publications. However, we have not found such obvious publications in this regard. An analysis of concomitant use of antidepressants indicated that, particularly in patients who did not take antidepressants previously, adding an antidepressant could be beneficial in increasing analgesic efficacy 21. There is no conclusive evidence published on QTP. In addition, it is important to remember that the use of multiple serotoninergic agents should be avoided or approached with caution because of the risk of serotonin syndrome. As for CNB, the evidence is inconsistent. There are meta-analyzes with positive results 22,23 and meta-analysis reviews with negative results 24.
Among the anesthetics and NMDA antagonists, the most used was the Lido IV, which stands out for its high frequency, followed by KTM. However, it is striking that there is occasional and relatively frequent use of PPF and SVN. The base of publications to support their use does not reach 50 % of respondents, which barely exceeds 80 % if those who respond using information from courses, conferences or extrapolation from basic sciences are added as a basis for endorsing their use. In this group, those based on their own experience for use account for 12 % of respondents. However, again, there are not many high-quality randomized controlled trials with NMDA receptor antagonists. The level of recommendation in terms of effectiveness for the NP is low 25. In fact, it is recommended to use them as an alternative option only for patients who do not respond to standard treatment.
Among the treatments of topical use, the use of Li5 % is overwhelmingly highlighted, followed by BTA. Off-label CPS and transmucosal lidocaine are used relatively frequently, more even than antiepileptic drugs, and at the same level as KTM or VLF. Both US and European guidelines already suggest the use of Li5 % in diabetic painful neuropathy and post-surgical pain 26. In the case of localized and superficial pain, topical treatment with Li5 % seems rational 26. In fact, Li5 % have been evaluated in a multicenter randomized European study to assess its efficacy and safety vs. PGB. 27. The patients included were refractory to systemic treatments, mostly consisting of anticonvulsants, antidepressants, and opioids. Li5 % were used as "complementary therapy" 28, i.e., as combination therapy. In order to evaluate the potential use of this topical treatment as an element of multimodal therapy, Casale et al conducted an open-label, prospective, observational study 26. One month after the start of therapy, a statistically significant clinical decrease was detected compared to the initial pain intensity (p < 0.0001). Another study was conducted to assess whether treatment with Li5 % was also effective in various forms and locations of polyneuropathy 29. This revealed that, as complementary therapy, Li5 % was clearly effective in reducing continuous pain (p = 0.017) and alodynia (p = 0.023).
For CPS, administered alone or in conjunction with systemic neuropathic analgesics, integrated data have been analyzed 30, and a single 60-minute treatment has been shown to reduce pain for up to 12 weeks, regardless of concomitant use of systemic neuropathic analgesics. Other topical therapies have also been evaluated by a pilot study, which provides initial information on the use of new topical preparations containing amitriptyline, KTM, and a combination of both in the treatment of NP 31. In those who used the combination cream, there was a statistically significant effect, with significantly greater analgesia and good tolerance without evidence of significant systemic absorption.
It is important to note that, except for antiepileptics, the percentage claiming to base their use on own experience exceeds 10 %. This is probably due to the lack of publications supporting the use of all these drugs, so clinicians need to rely on information obtained from courses, conferences and extrapolation of mechanisms of action. This shows once again both the need to communicate the results obtained in clinical practice, especially for off-label use, and the need to give continuity to the results obtained in the basic sciences, in a translational manner.
The most frequently used off-label treatments according to the responses obtained are, in order: Li5 %, BTA, LiIV and VLF. This is altered if used occasionally are added, the most used in this case are, in order: VLF, Li5 %, BTA and LiIV. It is probably because these are the ones with more evidence available. Still, there is little solid evidence to support the use of VLF in the NP 32. There are studies with methodological limitations and a considerable risk of biases that do not provide consistent or first-level evidence. The role of systemic local anesthetics in treating NP has been controversial and difficult to define objectively, even compared to placebo interventions. LiIV was more effective than placebo in decreasing NP in a meta-analysis with robust end results against statistical heterogeneity or clinical variability 33. It can be argued against that the difference in means of 11 mm in a VAS 0-100 (or 1.1 in an NRS 0-10) represents a clinical difference of small magnitude, but this size of the effect is clinically relevant for NP. In addition, most patients had previously been treated with other analgesics having failed. Therefore, this is a very difficult group to manage, and the small quantitative differences in these patients are valuable. LiIV can relieve NP in selected patients with evidence suggesting that this analgesic effect is clinically important, with good quality evidence 34. The recommended doses are from 5 mg/kg to 7.5 mg/kg body weight. However, for intravenous KTM, although the quality of evidence is also good, its application is not recommended as a clinically viable treatment option, unless performed in hospital settings under the care of a specialist 35. In fact, for repeated infusions in the complex regional pain syndrome (CRPS) the recommendation is inconclusive. In patients with NP, there is insufficient evidence to recommend intravenous ketamine as a long-term treatment strategy 34.
Finally, it should be noted that this article has a number of limitations. First, it is based on the description of a survey performed among the SED members, so it cannot be said that it represents the total medical group in Spain. Despite an announcement was published in the RESED, the selection bias cannot be ignored because the survey was sent only to the SED members. However, it can be argued that it can be representative of the group of members of this medical society. The second limitation is the low response rate among respondents. However, a very close response rate, although low, has been considered sufficient as a sample of a similar group 36. Other surveys have had similar response rates 37,38. Third, there is an overweight in the anesthesiology specialty, and it would be necessary to know more about other specialties. Perhaps it could be done by repeating the same survey in other societies in order to compare and add data. However, the results obtained serve as the basis for future studies or for improving the knowledge of physicians' prescribing habits of non-NP-indicated drug.
In conclusion, we are not aware of any prior information on the off-label use of drugs for NP. This would be the first time that a survey has been conducted to find out the prescribing habits in this area. Being the first, it cannot be compared. But it is striking that in several drug groups there is a discrepancy between the use of some drugs and the reasoning about that use based on publications, versus the publications currently available. We therefore believe that the information obtained, even having a relative weight, is of the utmost importance to understand the approach to the NP in our environment.
ACKNOWLEDGMENTS
The Neuropathic Pain Working Group of the Spanish Pain Society would like to thank all the members who answered the survey for their collaboration and dedication. Without their help, this article would not have been possible. Their participation will allow having a better understanding of the treatment needs for NP. Thank you.
REFERENCES
1. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630-5. DOI: 10.1212/01.wnl.0000282763.29778.59. [ Links ]
2. Bouhassira D. Définition et classification des douleurs neuropathiques [Definition and classification of neuropathic pain]. Presse Med. 2008;37(2 Pt 2):311-4. DOI: 10.1016/j.lpm.2007.07.022 [ Links ]
3. Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7(4):281-9. DOI: 10.1016/j.jpain.2005.11.008. [ Links ]
4. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654-62. DOI: 10.1016/j.pain.2013.11.013. Erratum in: Pain. 2014;155(9):1907. [ Links ]
5. Cherif F, Zouari H, Cherif W, Hadded M, Cheour M, Damak R. Depression prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Research and Management. 2020. Article ID 7408508. DOI: 10.1155/2020/7408508. [ Links ]
6. Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010-8. DOI: 10.1111/j.1468-1331.2010.02969.x. [ Links ]
7. Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599-606. DOI: 10.1097/j.pain.0000000000000492. [ Links ]
8. Binder A, Baron R. The Pharmacological Therapy of Chronic Neuropathic Pain. Dtsch Arztebl Int. 2016;113(37):616-25. DOI: 10.3238/arztebl.2016.0616. [ Links ]
9. Serpell MG; Neuropathic Pain Study Group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99(3):557-66. DOI: 10.1016/S0304-3959(02)00255-5. [ Links ]
10. Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. [ Links ]
11. Holbech JV, Jung A, Jonsson T. Combination treatment of neuropathic pain: Danish expert recommendations based on a Delphi process. J Pain Res. 2017;10:1467-75. DOI: 10.2147/JPR.S138099. [ Links ]
12. Smith BH, Torrance N, Ferguson JA, Bennett MI, Serpell MG, Dunn KM. Towards a definition of refractory neuropathic pain for epidemiological research. An international Delphi survey of experts. BMC Neurol. 2012;12:29. DOI: 10.1186/1471-2377-12-29. [ Links ]
13. Ciaramitaro P, Cruccu G, de Tommaso M, Devigili G, Fornasari D, Geppetti P, et al. A Delphi consensus statement of the Neuropathic Pain Special Interest Group of the Italian Neurological Society on pharmacoresistant neuropathic pain. Neurol Sci. 2019;40(7):1425-31. DOI: 10.1007/s10072-019-03870-y. [ Links ]
14. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-73. DOI: 10.1016/S1474-4422(14)70251-0. [ Links ]
15. Moulin DE, Boularge A, Clark AJ. Pharmacological management of chronic neuropathic pain: revised consensus statement from the canadian pain society. Pain Res Manag. 2014:19(6):328-35. [ Links ]
16. Attal N, Gruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-23. DOI: 10.1111/j.1468-1331.2010.02999.x. [ Links ]
17. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tölle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The "COMBO-DN study"-a multinational, randomized, double-blind,parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616-25. DOI: 10.1016/j.pain.2013.05.043. [ Links ]
18. Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12(11)1084-95. DOI: 10.1016/S1474-4422(13)70193-5. [ Links ]
19. Galvez R. Guía de Práctica Clínica sobre el Tratamiento Farmacológico del Dolor Neuropático Periférico en Atención Primaria. Guía de la Sociedad Española del Dolor (SED), la Sociedad Española de Médicos de Atención Primaria (SEMERGEN), la Sociedad Española de Medicina de Familia y Comunitaria (SEMFYC) y la Sociedad Española de Médicos Generales y de Familia (SEMG). Madrid: Ed Master Line & Prodigio SL; 2016. [ Links ]
20. Guan J, Tanaka S, Kawakami K. Anticonvulsivants in combination pharmacotherapy for treatment of neuropathic pain in cancer patients: a systematic review and meta-analysis. Clin J Pain. 2016;32(8):719-25. DOI: 10.1097/AJP.0000000000000310. [ Links ]
21. Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, non inferiority comparison. Mayo Clin Proc. 2011;86(7):615-26. DOI: 10.4065/mcp.2010.0681. [ Links ]
22. Aviram J, Samuelly-Leichtag G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician. 2017;20(6):E755-E796.. [ Links ]
23. Meng H, Johnston B, Englesakis M, Moulin DE, Bhatia A. Selective Cannabinoids for Chronic Neuropathic Pain: A Systematic Review and Meta-analysis. Anesth Analg. 2017;125(5):1638-52. DOI: 10.1213/ANE.0000000000002110. [ Links ]
24. Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, et al. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64(2):e78-e94. [ Links ]
25. Sumitani M, Sakai T, Matsuda Y, Abe H, Yamaguchi S, Hosokawa T, et al. Executive summary of the Clinical Guidelines of Pharmacotherapy for Neuropathic Pain: second edition by the Japanese Society of Pain Clinicians. J Anesth. 2018;32(3):463-78. DOI: 10.1007/s00540-018-2501-0. [ Links ]
26. Casale R, Polati E, Schweiger V, Coluzzi F, Bhaskar A, Consalvo M. Localized neuropathic pain-5% lidocaine medicated patch as a firstline treatment and as add-on therapy: literature review and personal experience. Minerva Med. 2014;105(3):177-95. [ Links ]
27. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5 % lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663-76. DOI: 10.1185/03007990903047880. [ Links ]
28. Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5 % patches: study of 40 consecutive cases. Curr Med Res Opin. 2009;25(11):2737-43. DOI: 10.1185/03007990903282297. [ Links ]
29. Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5 % in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106(1-2):151-8. DOI: 10.1016/s0304-3959(03)00317-8. [ Links ]
30. Irving GA, Backonja M, Rauck R, Webster LR, Tobias J, Vanhove GF. NGX-4010, a capsaicin 8 % dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin J Pain. 2012;28(2):101-7. DOI: 10.1097/AJP.0b013e318227403d. [ Links ]
31. Lynch ME, Clark AJ, Sawynok J. A pilot study examining topical amitriptiline, ketamine anda a combination of both in the treatment of neuropathic pain. Clin J Pain. 2013;19 (5)323-8. DOI: 10.1097/00002508-200309000-00007. [ Links ]
32. Gallager HC, Gallager RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database Syt Rev. 2015;23(8):CD011091. DOI: 10.1002/14651858.CD011091.pub2. [ Links ]
33. Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2005;101(6):1738-49. DOI: 10.1213/01.ANE.0000186348.86792.38. [ Links ]
34. Mailis A, Taenzer P. Evidence-based guideline for neuropathic pain interventional treatments: spinal cord stimulation, intravenous infusions, epidural injections and nerve blocks. Pain Res Manag. 2012;17(3):150-8. DOI: 10.1155/2012/794325. [ Links ]
35. Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154(11):2249-61. DOI: 10.1016/j.pain.2013.06.004. [ Links ]
36. Perelló Bratescu A, Adriyanov B, Dürsteler C, Sisó-Almirall A, Álvarez Carrera MA, Riera Nadal N. Strong opioids and non-cancer chronic pain in Catalonia. An analysis of the family physicians prescription patterns. Rev Esp Anestesiol Reanim. 2020;67(2):68-75. DOI: 10.1016/j.redar.2019.08.003. [ Links ]
37. Johnson M, Collett B, Castro-Lopes JM. The challenges of pain management in primary care: a pan-European survey. J Pain Res. 2013;6:393-401. DOI: 10.2147/JPR.S41883. [ Links ]
38. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375-82. DOI: 10.5055/jom.2014.0234. [ Links ]
Received: March 31, 2021; Accepted: July 12, 2021











 texto en
texto en