Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Enfermería Global
versión On-line ISSN 1695-6141
Enferm. glob. vol.20 no.62 Murcia abr. 2021 Epub 18-Mayo-2021
https://dx.doi.org/10.6018/eglobal.438931
Originals
Analysis of drug incompatibilities in a cardiac intensive unit: a cross-sectional study
1Specialist in Cardiovascular Nursing, by the Cardiovascular Nursing Residency Program at the State University of Rio de Janeiro. Brazil
2PhD in Nursing, Adjunct Professor in the medical-surgical department, Faculty of Nursing, State University of Rio de Janeiro, Rio de Janeiro, Brazil
Master in Nursing, Assistant Professor in the medical-surgical department, Faculty of Nursing, State University of Rio de Janeiro, Rio de Janeiro, Brazil.
Objective:
To evaluate the incompatibilities of intravenous medications in cardiac patients admitted to a cardiac intensive unit, associating possible incompatibilities with the severity and characteristics of the adverse event.
Method:
Cross-sectional, observational, and quantitative study, held in a Cardiac intensive Unit of a University Hospital in the city of Rio de Janeiro. Data collection took place from March to June 2018. Micromedex® identified and classified drug incompatibilities.
Results:
We analyzed 111 prescriptions with a total of 1,497 prescription drugs, the average number of prescription drugs was 13.49 (6 ± 24), 580 (38.74%) intravenously in which 41.38% were administered simultaneously with another medicine. The study showed 121 incompatibilities and the drug classes that had the highest number of incompatibilities were diuretics, hypnotics and sedatives, cardiovascular stimulants (vasoactive amines), antibiotics for systemic use, corticosteroids for systemic use, cardiovascular vasodilators, and antiarrhythmic agents. We highlight the incompatibilities classified as moderate, furosemide with hydrocortisone, and midazolam with omeprazole, and severe fentanyl with amiodarone.
Conclusion:
The study highlights the importance of medication scheduling and administration by the nursing team based on pharmacological knowledge. We expect that the chart of recommendations prepared in the study with nursing care related to incompatibilities with greater potential for severity and its events can contribute to drug safety.
Keywords: Drug incompatibility; Administration, Intravenous; Infusions, Intravenous, and Patient Safety
INTRODUCTION
The administration of medications is a usual activity in patients hospitalized in intensive cardiological therapies. Due to a large number of medications administered and the limited number of venous access routes, drug incompatibilities appear as a frequent problem in clinical practice 1.
Intravenous medications should be infused in an exclusive route for each medication. However, in clinical practice, most infusions are administered via a “Y” connector, whereby medications are mixed in the catheter lumen before reaching the bloodstream, which may favor the occurrence of drug incompatibilities 1.
Drug incompatibility is defined by an in vitro interaction as a result of a chemical reaction between the active ingredient and the component of another drug when combined in the same syringe, equipment, or bottle during preparation or administration 2,3.
The concomitant administration of incompatible drugs is considered a medication error, classified as an avoidable adverse event for patients undergoing infusional therapy 1.
According to the World Health Organization (WHO), medication errors cause at least one death per day and harm approximately 1.3 million people annually only in the United States. The cost associated with medication errors has been estimated at $ 42 billion per year worldwide or almost 1% of total global health expenditures 4.
In Brazil, a study with 104 prescriptions identified a total of 304 drug incompatibilities, with an average of 2.33 incompatibilities per prescription. We noticed that in 63% of the analyzed cases, drugs administered in bolus are incompatible with those of continuous administration 5.
Incompatibilities can have several consequences. A review study showed that among the main adverse events caused by drug incompatibility, the most reported were: ineffective therapy, which leads to longer hospital stays and higher hospital costs; the occlusion of the catheter that can lead to infections and the occurrence of thromboembolic events, caused by the precipitation of the medication that can cause death. In this sense, the multi-professional team must implement measures to avoid this problem 6.
This study aimed at the incompatibility of intravenous drugs in cardiac patients in an intensive care unit of a university hospital in the State of Rio de Janeiro (Brazil). In this sense, we elaborated the following research question: what are the main intravenous drug incompatibilities found in cardiac patients?
To answer the research question, we aimed to assess the incompatibilities of intravenous medications in cardiac patients hospitalized in an intensive care unit, associating the possible incompatibilities with the severity and characteristics of the adverse event.
MATERIAL AND METHOD
This is a descriptive study with a cross-sectional design of a non-participating observational nature with a quantitative approach of the data, following the twenty-two items of the STROBE statement 7,8. In this study, the primary outcome was to identify the main intravenous drug incompatibilities. We identified the incompatibilities through the patient's medical prescription and direct observation of the patient's intravenous infusion devices.
The research was carried out in a cardiac intensive unit, with nine beds at a public university hospital, located in the city of Rio de Janeiro. These beds had critically ill patients who need continuous assistance. Most of these patients use intravenous medications through peripheral venous access (together with a multipath extensor) or central venous catheter (mono, double or triple lumen). Nurses carried out and schedule the medical prescriptions daily.
The inclusion criteria in the study were cardiac patients, admitted to the cardiac intensive unit, with more than one venous medication prescribed and who had punctured venous access. The exclusion criteria were patients under 18; with prescriptions not scheduled by nurses and hospitalization time of fewer than 24 hours (aiming to allow the professional to have adequate planning for infusional therapy).
Data collection took place from March to June 2018 and was performed using an instrument developed by the researchers. The variables related to the objectives of the study included the identification of the medication (drugs in use, their medication classes, and the route of administration) and data on incompatibilities (type of infusion - intermittent or continuous, form of administration - exclusive or simultaneous, and classification of incompatibility).
To determine the sample size, we performed a sample calculation considering a population of 160 prescriptions/month, a sample error of 5%, and a maximum percentage of error of 30%, as indicated in the literature, obtaining a sample of 108 prescriptions.
The main researcher collected the data through direct observation of medication prescriptions and their administrations in hospitalized patients, using a collection instrument.
After collection, we tabulated the data in Microsoft Excel® and we did a quick/initial reading of the material to systematize and organize the data. Afterward, we imported these data into Micromedex® 9 for the identification and classification of drug incompatibilities. In this way, the data were investigated, quantified, and interpreted.
The data were analyzed based on descriptive statistics, inferential as mean, standard deviation, and confidence index to identify the most severe incompatibilities in the drugs used.
The research followed the determinations of Resolution 466/12 of the National Health Council and was approved by the institution's Research Ethics Committee (CEP) under nº 82001317800005259 on November 18, 2017.
RESULTS
We analyzed 111 prescriptions, which presented a total of 1,497 medications with an average of 13.49 (6 ± 24) medications per prescription. Intravenous was used on 580 (38.74%) and 917 (61.26%) had other administration routes.
A continuous administration was considered when performed by the infusion pump for more than one hour, and intermittent administration of medications that were infused in bolus, with fast administration, or within a period less than or equal to one hour.
The intravenous drugs identified in the prescriptions were characterized within the aspects related to administration to achieve the objective proposed by the study: type of infusion and form of administration, where they were identified, which drugs were administered simultaneously. To track them for potential incompatibility, 41.38% of the 580 intravenous drugs were administered simultaneously with another drug (Table 1).
Table 1. Characterization of intravenous drug administration observed in the prescriptions. Rio de Janeiro, RJ, Brazil, 2019. (n = 580)
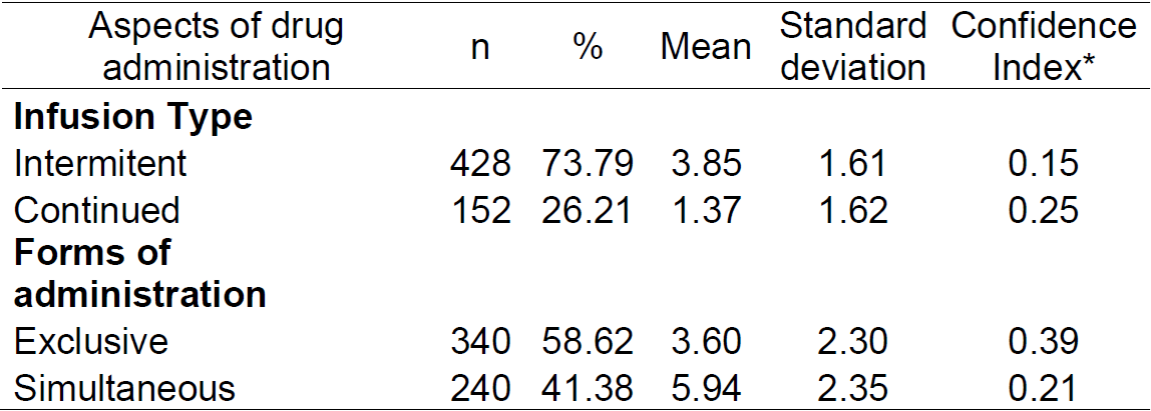
* 95% confidence index for the mean.
Then, to analyze the 240 intravenous drugs administered simultaneously, we initially chose to distribute them according to the doses administered, totaling 981 doses, classified according to Anatomical Therapeutic Chemical Classification System (ATS)/(WHO) 10, which divides medicines into different groups and subgroups according to the organ or system on which they operate and according to their chemical, pharmacological and therapeutic properties. To analyze the potential drug incompatibilities, we used Micromedex® software 9, and the compatible (284), incompatible (185), and untested (515) doses were identified, as shown in Table 2.
Table 2. Distribution of doses of drugs administered simultaneously and intravenously according to the ATC/WHO classification level and the incompatibilities observed in doses administered simultaneously. Rio de Janeiro, RJ, Brazil, 2019. (n = 981)
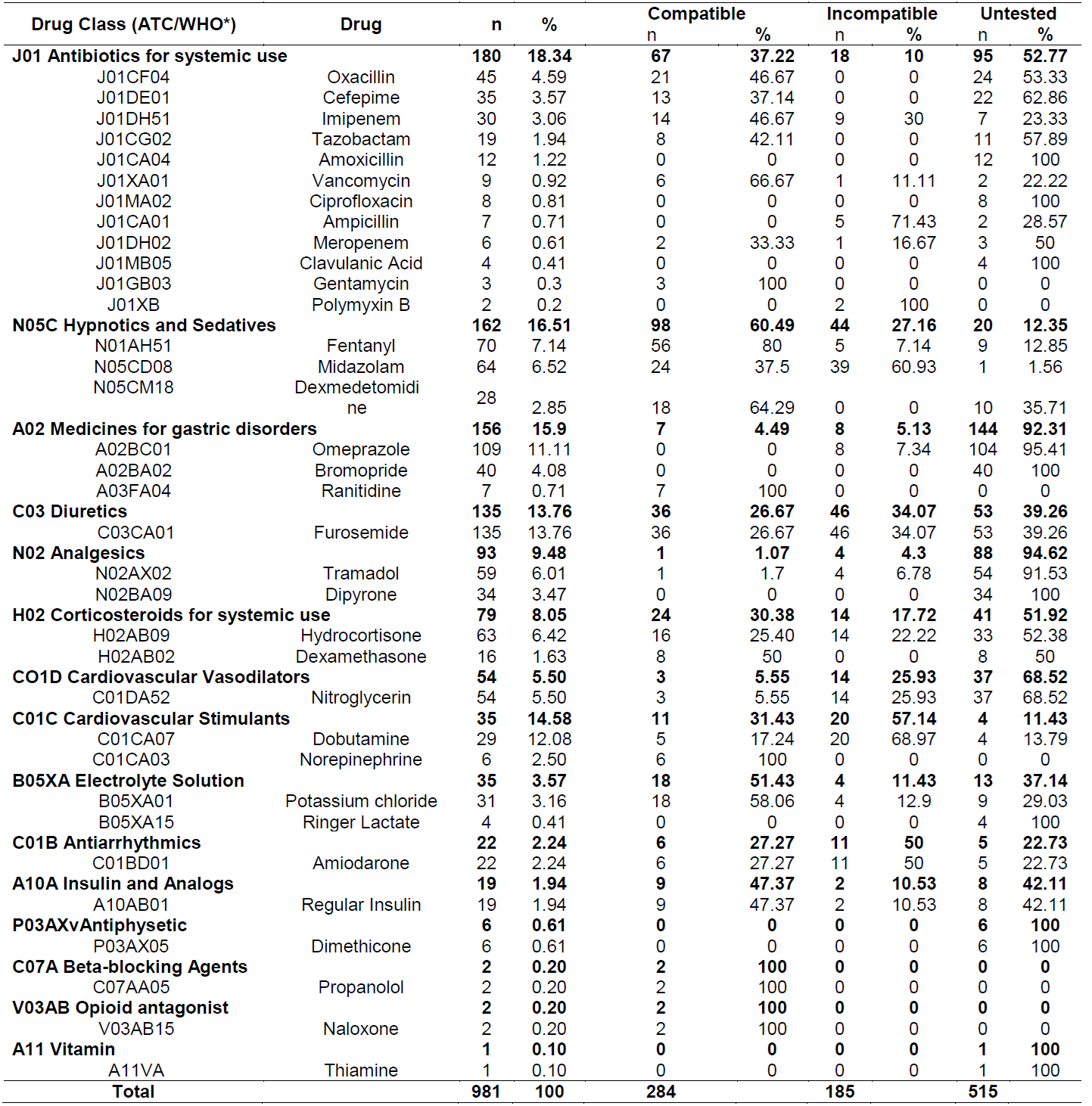
*ATC: Anatomical Therapeutic Chemical Classification System; WHO: World Health Organization 10.
Were found 14 therapeutic subgroups among the intravenous drugs for simultaneous administration, classified by the ATC. There was a higher frequency in the subgroups of antibiotics for systemic use, highlighting oxacillin, cefepime and imipenem, the hypnotics and sedatives, among them fentanyl and midazolam, among the drugs for gastric disorders omeprazole was administered more frequently, furosemide is the diuretic of choice in the unit studied and tramadol was the most used analgesic.
The drug classes that showed the greatest number of incompatibilities were: diuretics, hypnotics and sedatives, cardiovascular stimulants (vasoactive amines), antibiotics for systemic use, corticosteroids for systemic use, cardiovascular vasodilators, and antiarrhythmic agents.
The medications with the highest number of incompatibilities were Furosemide (46 - 34.07%), Midazolam (39 - 60.94%), Dobutamine (20 -68.97%) Hydrocortisone (14 -22.22%), Nitroglycerin (14 -26.42%), and Amiodarone (11 -52.39%).
The study identified 185 (one hundred and eighty-five) incompatible doses, but when excluding repeated doses, we found a total of 121 drug incompatibilities.
To perform the classification of the severity of the adverse event, we followed the definitions of Micromedex®. We consider a ‘severe’ event when the incompatibility is fatal and/or require medical intervention to minimize or prevent severe adverse effects. A 'moderate' event is when the incompatibility can result in an exacerbation of the patient's condition and/or require a change in therapy. Drug incompatibility as 'mild' is when the interaction would have limited clinical effects. Manifestations may include an increase in the frequency or severity of side effects, but they would generally not require a major change in therapy.
Medicines for which information is not available were classified as 'untested'; in these cases, simultaneous administration should be avoided, if possible.
Table 3 shows the classification of incompatible medications for the number of doses administered and their severity.
Table 3. Description of the drug incompatibilities found and their classifications regarding severity Rio de Janeiro, RJ, Brazil 2019. (n = 121)
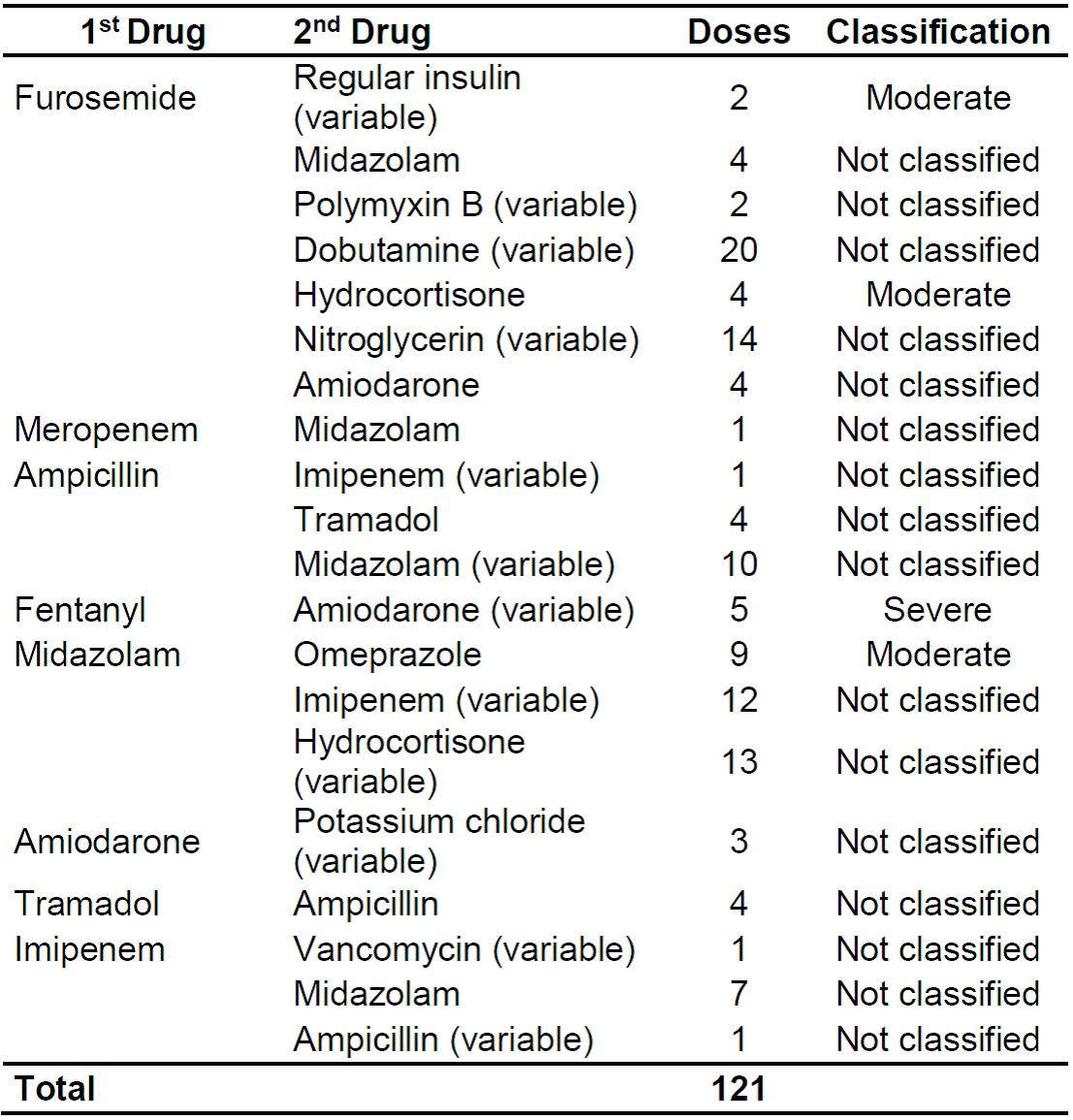
We highlight incompatibilities with greater potential for severity such as moderate in furosemide x hydrocortisone and midazolam x omeprazole, and severe in fentanyl x amiodarone.
DISCUSSION
Incompatibilities related to the administration of simultaneous medications are a major problem, especially in intensive care where administration of intravenous medications is part of daily clinical practice 11,12.
In this study, the mean of 13.49 drugs per prescription shows a large number of drugs per patient, showing a profile of poly-pharmaceutical patients in the cardiac intensive unit. The presence of polypharmacy and intravenous administration are indicators that reflect the severity of the population studied and are risk factors for the occurrence of drug incompatibility 13.
Ratifying the occurrence of polypharmacy in intensive care units, a study carried out with critically ill patients presented similar results in 3 months. it identified 1,019 prescription drugs with a mean of 10.2 ± 3.4 drugs per prescription 1.
To prevent incompatibilities in poly-pharmaceutical patients, the nurse has the challenge of scheduling the drugs and managing infusional therapy to reduce adverse events not only with incompatibilities but also with drug interactions. In practice, the patient is exposed to the risk of drug incompatibilities when he has a high number of prescribed drugs and greater than the capacity for exclusive administration. The use of more than six drugs per day increases the risk of drug interactions 9.8 times and the prevalence of incompatibilities is strongly associated with the number of drugs prescribed 14-16.
Another study analyzed 100 prescriptions and found 68% of prescriptions to be at least incompatible. The study evaluated 1,854 drug combinations with 271 incompatible combinations (14.6%), 372 untested (20.0%) and 1,211 were compatible (65.4%). A mean of 4.0 ± 3.3 incompatibilities was observed per prescription (average) obtained in the 68 (sixty-eight) prescriptions that presented drug incompatibilities 1.
The results of this research showed that most medications were infused through central venous access, 62 (55.86%). We observed that among patients using central venous catheters, most catheters were double-lumen. The use of multi-lumen catheters is a relevant strategy for the prevention of incompatibilities, as it allows different intravenous drugs to be administered separately, but at the same time 1.
The most frequent incompatibilities in this study were between furosemide and dobutamine (16.53%), Furosemide and Nitroglycerin (11.57%), Midazolam, and Hydrocortisone (10.74%), Midazolam and Imipenem (9.92%), and Ampicillin with Tramadol (8.26%). However, these incompatibilities were not classified according to their severity by Micromedex®. All medications are extremely important and highly usable in cardiac intensive units, which confirms the attention that nurses should have in the process of scheduling and administering them 15,16.
Another study identified that the most frequent incompatibilities were between midazolam and hydrocortisone (8.9%), cefepime and midazolam (5.2%), and hydrocortisone and vancomycin (5.2%). The studies show a profile of patients and similar drugs and the incompatibility between drugs such as midazolam and hydrocortisone are highlighted in both studies. However, this study has a slightly higher prevalence of 10.74% between midazolam and hydrocortisone, while in another study1 we found a prevalence of 8.9%, very close results, showing the potential for incompatibility of these drugs and emphasizing the importance of a different look at scheduling and administration 1.
We could not analyze the drug bromopride and sodium dipyrone due to the absence of this item in the software used. However, the manufacturer warns of the possibility of incompatibility and recommends that dipyrone sodium should not be administered with other injecTable drugs 17. This study also identified many drugs that were untested for drug incompatibility, which shows the deficiency in the knowledge of incompatibilities and the need for further studies on the subject.
Also, among the identified incompatible doses, most (75.21%) were not classified according to the severity of the event. However, it is still important to be careful during the scheduling and administration of these medications since, besides the severity of the event, it should be avoided.
Among the incompatible doses classified as severe, fentanyl with amiodarone stands out. Concomitant use of these drugs can result in cardiac toxicity (low cardiac output) and an increased risk of fentanyl toxicity (CNS depression, respiratory depression). The nurse must monitor cardiovascular complications, discuss the adjustment of the dose or suspension of one or both drugs with the multidisciplinary team. The concomitant use of amiodarone and fentanyl can cause high plasma concentrations of fentanyl, which can cause excessive sedation and respiratory depression 16,17.
The systemic reactions caused by sedatives are potentially dangerous, most of them of a cardiorespiratory nature. The most common are hypoventilation, hypertension, hypotension, hypoxia, tachycardia, bradycardia. Some can be enhanced by the pain and discomfort of patients, requiring greater doses of sedatives, worsening hypoxia, and arrhythmia, which can lead to cardiac arrest 17.
Among the incompatibilities classified as moderate, there are 2 (1.65%) doses of furosemide simultaneously with regular insulin, 4 (3.31%) doses of furosemide with hydrocortisone, and 9 (7.44%) doses in administration simultaneous use of omeprazole with midazolam.
Concomitant use of furosemide and regular insulin may result in an increased risk of hyperglycemia; the increased need for insulin. Therefore, when the patient uses these two drugs simultaneously, the nurse must monitor the glucose levels more frequently, including the removal of the diuretic 14. Concomitant use of furosemide and hydrocortisone may result in hypokalemia. The potassium balance must be carefully monitored by the multidisciplinary team if administration occurs simultaneously. Concomitant use of midazolam and omeprazole may result in benzodiazepine toxicity (CNS depression, ataxia, lethargy) 16.
Insulin was also highlighted in other studies as well. Fourteen of 840 pairs of drugs identified were related to insulin, with 6.67% of the frequency of interaction involving this drug 15.
With these findings, nurses need to know the profile of patients and drug therapy in the unit studied according to the medication classes and incompatibilities, which allows subsidies to plan and guide behaviors for drug safety.
A study that addresses the knowledge of nursing professionals about drug interactions showed that the team had insufficient knowledge about drug interactions 18. Nurses must know about pharmacology to discuss with the multidisciplinary team the possibility of replacing drugs with a compatible therapeutic alternative, or about the indication of a venous catheter that offers a greater number of routes 15,18. When administering two or more simultaneous medications, the nurse must check if they are physically compatible, since chemical reactions require more contact time for a significant reduction in the concentration of the drug to occur 16,18,19, and these nurses' actions reflect safe and qualified care.
Another safety measure is the routine presence of the clinical pharmacy in intensive care units 16, contributing to the reduction of drug incompatibility by guiding the nursing team in the face of some doubts that may arise during the stages of intravenous therapy.
As the nursing team is directly involved in the medication administration process, it needs fast and accurate information at the time of administration to prevent incompatibilities and ensure the effectiveness of the prescribed medication therapy, contributing to the therapeutic success and patient safety 14,15,20.
A quick consultation tool for administration in Y is another way to prevent this occurrence, considering that it is one of the items that are part of the nine items for the safe administration of medications 20.
With these results, the study produced a chart (Chart 1) as a product describing the most frequent incompatibilities and the respective adverse events that can be caused and outlined nursing care to minimize damage or prevent the event caused by the incompatibility.
Chart 1. Description of the drug incompatibilities found with the classifications referring to severity, describing which events and behaviors should be taken. Rio de Janeiro, RJ, Brazil 2019.
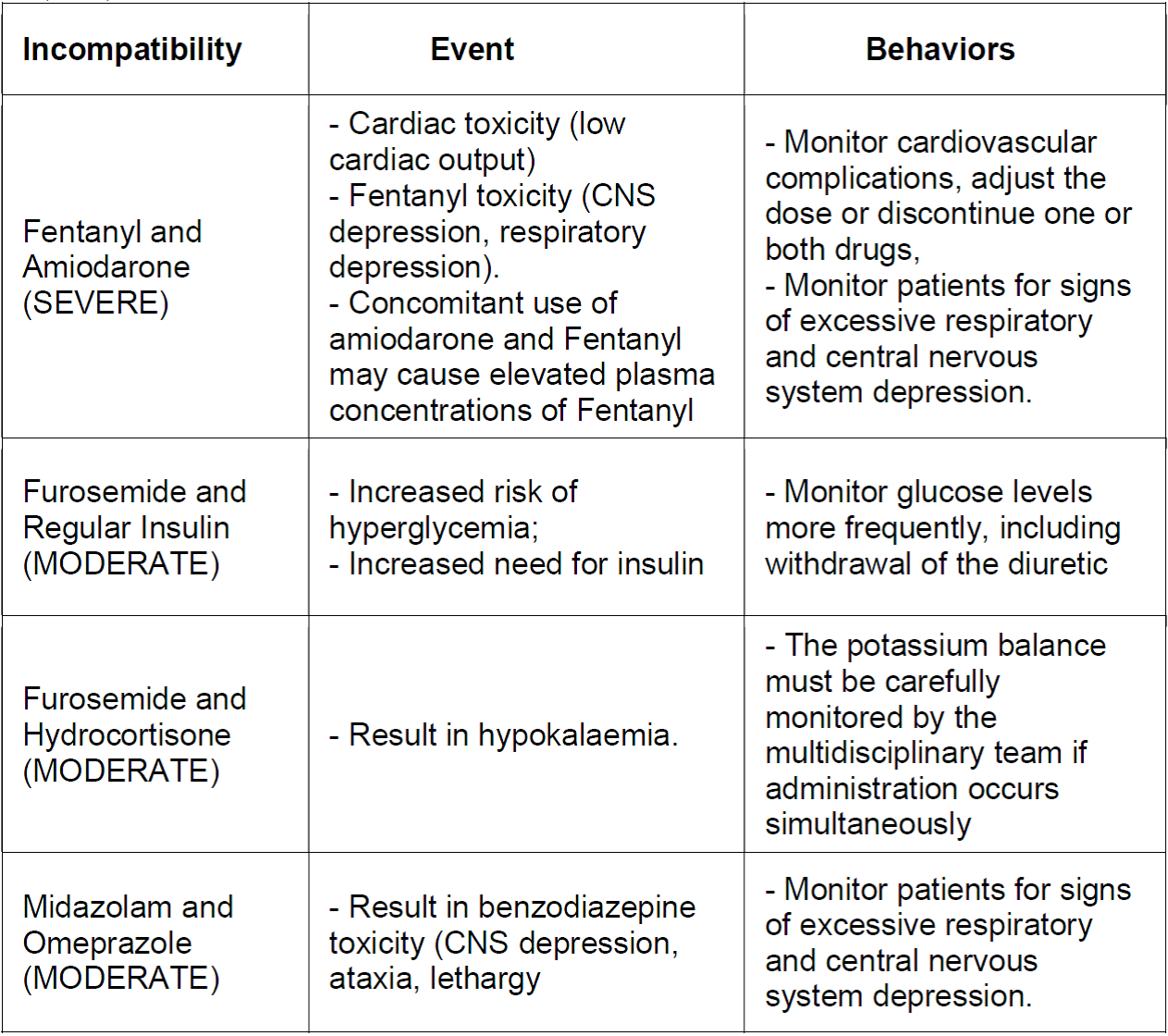
Source: The authors, 2019.
These strategies are very important to the nurses' scope of knowledge, emphasizing the importance of attention during the scheduling and administration of these medications since regardless of the severity classification of the event, it must be avoided to mitigate the risks.
CONCLUSION
The study shows a large number of prescription drugs (13,49) and 14 different drug classes, showing a profile of critical patients and polypharmaceuticals in the cardiac intensive unit.
Through this study, we observed that the medications with the highest number of incompatibilities were Furosemide, Midazolam Dobutamine, Hydrocortisone, Nitroglycerin, and Amiodarone.
We observed that 4.13% of the doses were classified as severe, 12.40% were classified as Moderate and 75.21% of the doses that presented incompatibility were not classified according to the severity of the event.
We identified that incompatibilities classified as severe and moderate can generate cardiac toxicity, such as low cardiac output, sedative toxicity (CNS depression, respiratory depression, hypoventilation, hypertension, hypotension, hypoxia, tachycardia, and bradycardia), Arrhythmias, which can cause also a cardiac arrest.
Thus, the importance of an assessment about the health status of each patient hospitalized in the cardiac intensive units is carried out, and the observation of the number of prescribed drugs and their particularities to provide a better choice of the infusion device and the number of lumens, reducing the risk of incompatibilities and providing a safer drug therapy.
We expect that the product elaborated in the study, an instruction with the most frequent incompatibilities, the respective adverse events that can be caused, and the most important nursing care to minimize the damage or the prevention of the event caused by the incompatibility, can contribute to drug safety at the study place and be multiplied and adapted to other units with the same characteristics.
There is still a significant number of untested drug combinations and unclassified event severities, highlighting the need for further in-depth studies on drug safety.
Some limitations of this study are the software limitation due to the large number of untested medications related to incompatibilities, not presenting the event severity classification.
REFERENCIAS
1. Marsilio N, Silva D, Bueno D. Incompatibilidades medicamentosas em centro de tratamento intensivo adulto de um hospital universitário. Rev Bras Ter Intensiva. Porto Alegre. 2016; 28(2)147-153. DOI: http://dx.doi.org/10.5935/0103-507X.20160029 [ Links ]
2. Cerdá S, Palau M, Nicolau R, Rubert M, Juan E. Administración compatible de la terapia intravenosa continua em el paciente coronário crítico. Enferm Cardiol. España, 2013; 59(2): 46-49. [ Links ]
3. Leal K, Leopoldino R, Verissimo L. Potencial de incompatibilidade de medicamentos intravenosos em uma unidade pediátrica. Einstein. Natal, 2016;14(2):185-9. DOI: https://doi.org/10.1590/S1679-45082016AO3723 [ Links ]
4. World Health Organization. Medication Without Harm - Global Patient Safety Challenge on Medication Safety. Geneva: World Health Organization; 2017. [acess 2020 jul 06]. Available from: https://www.who.int/patientsafety/medication-safety/medication-without-harm-brochure/en/ [ Links ]
5. Prelhacoski D, Silva D, Comarella L. Incompatibilidade medicamentosa em Unidade de Terapia Intensiva Pediatrica. UNIANDRADE. Paraná, 2016; 16(2):73-81. DOI: http://dx.doi.org/10.18024/1519-5694/revuniandrade.v16n2p73-81 [ Links ]
6. Paixão, FM, Camerini, FG, Fassarella, CS, Henrique, DM, Assad, LG, Radighieri, AR. Gravidade das incompatibilidades medicamentosas em pacientes críticos: Uma revisão integrativa. Saúde Coletiva (Barueri), 2019; 9(51):1907-1912.DOI: https://doi.org/10.36489/saudecoletiva.2019v9i51p1907-1912 [ Links ]
7. Malconi M, Lakatos E. Fundamentos de metodologia científica. 7. Ed-São Paulo: Atlas, 2010. [ Links ]
8. Malta M, Cardoso LO, Bastos FI, Magnanini MMF, Silva CMFP. Iniciativa STROBE: subsídios para a comunicação de estudos observacionais. Rev. Saúde Pública. 2010;44( 3 ): 559-565. DOI: https://doi.org/10.1590/S0034-89102010000300021 [ Links ]
9. Micromedex(r) 2.0. Truven Health Analytics Inc. 2013. [acesso em 10 out 2019]. Available from: http://www.micromedexsolutions.com [ Links ]
10. World Health Organization (WHO). WHO Collaborating Centre on Drug Statistics Methodology. ACT/DDD Index 2019 [Internet]. Oslo, Noruega: WHO; 2019. [cited 2020 Apr 26]. Available from: http://www.whocc.no/atc_ddd_index/ [ Links ]
11. Ondrej M, Jan M, Ales K, Jiri V. Incidence of intravenous drug incompatibilities in intensive care units. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2015; 159(4):652-656. DOI: https://doi.org/10.5507/bp.2014.057 [ Links ]
12. Benlabed M, Perez M, Gaudy R, et al. Clinical implications of intravenous drug incompatibilities in critically ill patients. Anaesth Crit Care Pain Med. 2019;38(2):173-180. DOI: https://doi.org/10.1016/j.accpm.2018.04.003 [ Links ]
13. Santos L, Martinbiancho J, Tadiotto A, Kreutz L. Perfil das interações medicamentosas solicitadas ao Centro de Informações sobre Medicamentos de hospital universitário. Clinical and Biomedical Research [Internet]. 2011 [acesso em 27 jul 2020];31(3):326-35. Avaible from: http://seer.ufrgs.br/hcpa/article/view/22183. [ Links ]
14. Paes GO, Moreira SO, Moreira MB, Martins TG. Incompatibilidade medicamentosa em terapia intensiva: revisão sobre as implicações para a prática de enfermagem. Rev. Eletr. Enf. 2017; 19. DOI: http://dx.doi.org/10.5216/ree.v19.38718 [ Links ]
15. Cortes ALB, Silvino ZR. Fatores associados a interações medicamentosas potenciais em um Centro de Terapia Intensiva: estudo transversal. Esc. Anna Nery . 2019; 23( 3 ): e20180326. DOI: http://dx.doi.org/10.1590/2177-9465-ean-2018-0326 [ Links ]
16. Scrignoli CP, Teixeira VCMC, Leal DCP. Drug interactions among the most prescribed drugs in adult intensive care unit. Rev Bras Farm Hosp Serv Saude [Internet]. 2019. [citado em 2 de abril de 2020];7(2). Avaible from: https://rbfhss.org.br/sbrafh/article/view/252. [ Links ]
17. Agência Nacional de Vigilância Sanitária (Brasil). Bulário eletrônico da Anvisa. [acesso em 20 out 2018]. Avaible from: http://www.anvisa.gov.br/bularioeletronico/ [ Links ]
18. Santos MDP et al. Conhecimento de profissionais de enfermagem de um hospital público sobre interações medicamentosas. Uningá Review. 2016; .28 (1):39-44. [acesso em 20 out 2018]. Avaible from: http://revista.uninga.br/index.php/uningareviews/article/view/1848/1448 [ Links ]
19. Bertsche T, Veith C, Stahl A, Hoppe-Tichy T, Meyer FJ, Katus H, et al. A purging procedure for pantoprazole and 4lumen catheters to prevent IV drug incompatibilities. Pharm World Sci. 2010; 32:663-669. DOI: http://dx.doi.org/10.1007/s11096-010-9422-9 [ Links ]
20. Negeliskii C. Efeito de uma intervenção educativa com profissionais de enfermagem acerca da segurança do paciente na administração de medicamentos injetáveis. [acesso em 20 out 2019]. Avaible from: Porto Alegre, 2015. https://proqualis.net/sites/proqualis.net/files/000978150.pdf [ Links ]
Received: July 30, 2020; Accepted: December 21, 2020











 texto en
texto en 


