Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.5 sep. 2007
Maxillary necrosis by mucormycosis. A case report and literature review
Ajit Auluck
MDS. Assistant Professor. Oral Medicine and Radiology. Manipal college of Dental Sciences, Mangalore, India
ABSTRACT
The maxilla rarely undergoes necrosis due to its rich vascularity. Maxillary necrosis can occur due to bacterial infections such as osteomyelitis, viral infections such as herpes zoster or fungal infections such as mucormycosis, aspergillosis etc. Mucormycosis is an opportunistic fulminant fungal infection, which mainly infects immunocompromised patients. The infection begins in the nose and paranasal sinuses due to inhalation of fungal spores. The infection can spread to orbital and intracranial structures either by direct invasion or through the blood vessels. The fungus invades the arteries leading to thrombosis that subsequently causes necrosis of hard and soft tissues. We report a case of maxillary necrosis by mucormycosis in an uncontrolled diabetic patient to emphasize early diagnosis of this potentially fatal fungal infection. We briefly discuss different diseases which can lead to maxillary necrosis and review the current concepts in management of mucormycosis. Early diagnosis and prompt treatment can reduce the mortality and morbidity of this lethal fungal infection.
Key words: Maxillary bone necrosis, mucormycosis, uncontrolled diabetes.
Introduction
Mucormycosis is one of the most rapidly progressing and lethal form of fungal infection in humans which usually begins in the nose and paranasal sinuses (1). This fungus invades the arteries, forms thrombi within the blood vessels that reduce blood supply and cause necrosis of hard and soft tissues (1,2). Once entered into the arteries, the fungus can spread to orbital and intracranial structures (3,4). Usually mucormycosis presents as an acute infection and manifests as rhinocerebral, pulmonary, gastrointestinal, cutaneous or disseminated form (1). In the case presented here the infection followed a chronic course, and somewhat indolent form which eventually caused maxillary necrosis.
Case report
A man of 58 years reported for evaluation of pain in the right maxillary posterior region since 4 months previously. Pain was moderate in nature, aggravated on bending the head and chewing food. The patient also complained of nasal congestion and headache. There was no history of fever, purulent discharge, paraesthesia or foul odor.
The patient had undergone extraction of right maxillary first, second and third molars 6 months earlier due to poor periodontal health. Following extractions the socket never healed completely. Patient had persistent pain and discomfort for the last 6 months.
The patient was a known diabetic on treatment with oral hypoglycemics for the last 10 years. He had been under medical supervision for the first 3 years (Tab. PIO-G, Indi pharma). However, he continued the same medication (Patient also took ayurvedic/ herbal medications) for the last 7 years without consulting a physician or undergoing any routine blood sugar estimation. Due to financial constrains the patient did not take the medication as per the prescribed dose and avoided laboratory investigations.
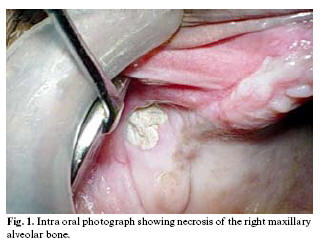
On general examination vital signs were within normal limits. Intraoral oral examination showed a necrotic bone of about 1 cm diameter in right maxillary molar region (fig-1). Maxillary molars were missing and surrounding soft tissues were normal.
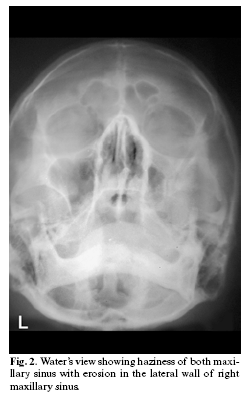
A radiograph (paranasal sinus view) was advised that showed haziness of right maxillary sinus with erosion of lateral sinus wall (fig-2). CT scan was not performed due to financial constrains. Biochemical investigations revealed an elevated blood sugar levels. Fasting blood sugar was 238 mg/dl (normal 60-90 mg/dl) & post prandial blood sugar level was 386 mg/dl (normal 90-140 mg/dl). A biopsy was advised. Hard tissue specimen along with the adjacent soft tissue was excised under local anesthesia and sent for histopathological examination.
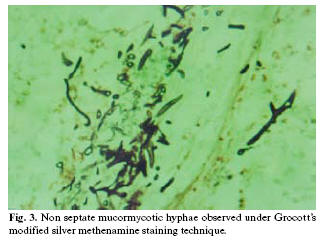
Histopathological examination with hematoxylin and eosin stain readily identified non-septate mucormycotic hyphae. Grocotts modified silver methenamine special staining technique further identified these non-septate branching hyphae of mucormycosis (fig 3). The culture report was non contributory.
The patient was hospitalized and physicians controlled blood sugar levels with insulin. The necrotic bone along with 1 cm of adjacent bone was excised under general anesthesia. The patient was administered Amphotericin-B 0.8mg/kg/day intravenously for two weeks. It was slowly infused over 4-6 hours and blood urea and creatinine levels were monitored as the drug can cause renal toxicity. Two weeks later the area started healing and subsequently after three months an obturator was made for the patient.
Discussion
Opportunistic fungal infections such as mucormycosis usually occur in immunocompromised patients but can infect healthy individuals as well(2-5). The predisposing factors for mucormycosis are uncontrolled diabetes (particularly in patients having ketoacidosis), malignancies such as lymphomas and leukemias, renal failure, organ transplant, long term corticosteroid and immunosuppressive therapy, cirrhosis, burns, protein energy malnutrition and AIDS. Our patient had uncontrolled diabetes which is a well known predisposing factor for mucormycosis(1-8).
Mucormycosis (Zygomycosis, phycomycosis) is an acute opportunistic infection caused by a saprophytic fungus that belongs to the class of phycomycetes. Although several genera are associated with this disease, the most common forms are Rhizopus, Rhizomucor and Absida. Rhizopus is the predominant pathogen accounting for 90% of the cases of rhinocerebral mucormycosis. This microbe may be cultured from the oral cavity, nasal passages, throat and stool of healthy patients without clinical signs of infection.
Uncontrolled diabetes mellitus can alter the normal immunologic response of patients to infections. Such patients have decreased granulocyte phagocytic ability with altered polymorphonuclear leukocyte response. Reports have suggested that the ability of serum of immunocompromised patients to inhibit Rhizopus invitro is reduced, which makes them suitable hosts to opportunistic fungal infections(8).
This fungal infection usually originates from the paranasal sinuses. The fungus invades the blood vessels and subsequently spreads through them. Once fungal hyphae enter into the blood stream they can disseminate to other organs such as cerebrum or lungs which can be fatal for the patient. Mucor hyphae form thrombi within the blood vessels that reduce vascularity to the tissues and cause necrosis(2-5,8). Peripheral vascular disease (due to microangiopathy & atherosclerosis) in diabetic patients also causes local tissue ischemia and increased susceptibility to infections (9) (Table 1). Therefore thrombosis of the internal maxillary artery or descending palatine artery caused by mucormycotic infection(4) as well as chronic diabetes in this patient had resulted in necrosis of the maxilla.
Usually mucormycosis occurs as a pulmonary, gastrointestinal, disseminated or rhinocerebral infection(6-10). In our patient, infection was only localized to the maxilla and it underwent necrosis without any other symptoms. Disseminated involvement of mucormycosis is observed in diabetics with ketoacidosis, which favors rapid proliferation of fungus and its invasion into the orbit and cerebrum(6). Mucormycosis is aggressive and potentially fatal in diabetic patients because of impaired host defense mechanism and increased availability of micronutrients such as iron(9). The general health of this patient was good and he did not developed ketoacidosis which facilitates spread of infection to other organ systems. Therefore, in this case the patient had a localized rhino-maxillary form of the disease which is a subdivision of well documented rhinocerebral mucormycosis (10,11). However the infection may spread to involve the cranium, orbit and other organs. Therefore, a team of specialists including a dentist, ophthalmologist, neurosurgeon and maxillofacial surgeon are required for management of such patients.
In the early stages of the disease, patients exhibit facial cellulites, anesthesia, nasal discharge, necrotic turbinates, fever, headache and lethargy(6). However, in contrast to other reports many of these symptoms were absent in this patient. He only had nasal congestion and headache.
Among the clinical differential diagnosis we can consider squamous cell carcinoma of maxillary sinus. Such cases present as chronic ulcers with raised margins causing exposure of underlying bone. A malignant salivary gland tumor arising from the accessory glands of the palate can also be considered in the differential diagnosis. Other features seen in cases of antral carcinoma are local pain, swelling, epistaxis, nasal discharge, epiphora, diplopia or numbness. In this patient there were no symptoms suggestive of any malignancy.
Extranodal NK T-cell lymphoma (nasal type angiocentric lymphoma or midline lethal granuloma) characteristically occurs in midline, affecting the oronasal region. In the initial stages patients may report nasal stuffiness, pain and palatal swelling. Later, patients develop progressive areas of ulceration that can lead to bone necrosis and perforation.
Wegeners granulomatosis is an uncommon condition characterized by a necrotizing granulomatous condition of respiratory tract, widespread vasculitis and necrotizing glomerulonephritis. Common presenting signs and symptoms include sinusitis, rhinorrhea, nasal stuffiness and epistaxis with or without complain of fever, arthralgia and weight loss. Gingiva has a peculiar erythematous hyperplasia and is termed strawberry gingivitis. There may be destruction of underlying palatal and alveolar bone causing oral-antral fistula.
Bone necrosis can also occur due to extension of infections such as acute necrotizing ulcerative gingivitis (ANUG) from the gingiva to bone. In the case reported here the gingiva was normal. Recent reports have suggested that jaw necrosis can also occur in patients on bisphosphonate therapy(12). Our patient did not report any such drug intake.
There is a close histopathological resemblance between mucormycosis and aspergillosis. Microscopically, aspergillosis has septate branching hyphae, which can be distinguished from mucormycotic hyphae by a smaller width and prominent acute angulations of branching hyphae(9,10). Moreover, the organism produces conidiophores, which were absent in this case. A definitive diagnosis of mucormycosis can be made by tissue biopsy that identifies the characteristic hyphae, by positive culture or both. Initial culture of diseased tissue may be negative and histopathologic examination is essential for early diagnosis(9) In this case, the fungus was identified by hematoxylin and eosin stain and confirmed by Grocotts silver methenamine special staining technique.
Three principles in the patient management were followed. Firstly, control of diabetes for which the patient was advised insulin therapy and dietary restrictions. Secondly, removal of the necrotic bone, which acted as a nidus of infection and prevented action of systemically, administered antifungal drugs (due to thrombosis of blood vessels). In this patient entire necrotic bone was removed along with the antral lining and the area was débrided with Betadine. Lastly, amphotericin B was administered parenterally as it is the drug of choice in treatment of mucormycotic infection(2,5,10). Blood urea & creatinine levels were monitored. Post-operatively patient was advised an obturator to prevent oronasal regurgitation.
Mucormycosis was long regarded as a fatal infection with poor prognosis. However with early medical and surgical management survival rates are now thought to exceed 80%(5). This patient survived because of an early diagnosis and prompt treatment.
In conclusion, an immunocompromised or immunosuppressed patient having bone necrosis following tooth extraction should alert a clinician of possible mucormycotic infection.
References
1. Leitner C, Hoffmann J, Zerfowski M, Reinert S. Mucormycosis: necrotizing soft tissue lesion of the face. J Oral Maxillofac Surg 2003;61:1354-8. [ Links ]
2. Pogrel MA, Miller CE. A case of maxillary necrosis. J Oral Maxillofac Surg 2003;61:489-93. [ Links ]
3. Zapico ADV, Suarez AR, Encinas PM, Angulo CM, Pozuelo EC. Mucormycosis of the sphenoidal sinus in an otherwise healthy patient. Case report and literature review. J Laryngol Otol 1996;110:471-3. [ Links ]
4. Jones AC, Bentsen TY, Fredman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol 1993;75: 455-60. [ Links ]
5. Salisbury PL 3rd, Caloss R Jr, Cruz JM, Powell BL, Cole R, Kohut RI. Mucormycosis of the mandible after dental extractions in a patient with acute myelogenous leukaemia. Oral Surg Oral Med Oral Pathol Oral Radiol. Endod 1997; 83:340-4. [ Links ]
6. Buhl MR, Joseph TP, Snelling BE, Buhl L. Temporofacial zygomycosis in a pregnant woman. Infection 1992; 20:230-2. [ Links ]
7. Napoli JA, Donegan JO. Aspergillosis and necrosis of maxilla: a case report. J Oral Maxillofac Surg 1991;49: 532-4. [ Links ]
8. Brown OE, Finn R. Mucormycosis of the mandible. J Oral Maxillofac Surg 1986;44:132-6. [ Links ]
9. Tugsel Z, Sezer B, Akalin T. Facial swelling and palatal ulceration in a diabetic patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:630-6. [ Links ]
10. Martin S Greenberg. Ulcerative vesicular and bullous lesions. In: Greenberg MS, Glick M eds. Burkets Oral Medicine Diagnosis and treatment. India: Elsevier; 2003.p.79. [ Links ]
11. Hazarika P, Ravikumar V, Nayak RG, Rao PS, Shivananda PG. Rhinocerebral mycosis. Ear Nose Throat J 1984;63:464-8. [ Links ]
12. Farrugia MC, Summerlin DJ, Krowiak E, Huntley T, Freeman S, Borrowdale R et al. Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope 2006;116:115-20. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Ajit Auluck
Department of Oral Medicine and Radiology
Manipal College of Dental Sciences
Light House Hill Road
Happankata
Mangalore 575001
Karnataka, India
Email: drajitauluck@gmail.com
Received: 26-07-2006
Accepted: 14-01-2007