INTRODUCTION
Topical gel drug administration is a localized drug delivery system anywhere in the body through ophthalmic, rectal, vaginal and skin as topical routes.1 Skin is one of the largest route of drug delivery action and readily accessible organs on human body for topical administration and is main route of topical drug delivery system. Topical application of drugs of potential advantages of delivering the drug directly to the site of action and acts for a long period of time.2,3
Lidocaine hydrochloride is the first amino amide type of local anesthetics and has been in use for many years. In dentistry, it is a drug of choice to temporarily anesthetize the tiny nerve endings located on the surfaces of the oral mucosa. As a local anesthetic, lidocaine is characterized by a rapid onset of action and intermediate duration of efficacy, making it suitable for infiltration and nerve block anesthesia.4 Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Skin penetration enhancers reversibly decrease the barrier resistance of the stratum corneum and allow drugs to penetrate more readily to the viable tissues and the systemic circulation5), (6. Penetration enhancers are applied to improve the permeation of the poor permeable drug through the skin. They do not have any therapeutic effect but they enhance the penetration of drugs across the membrane.
Nowadays, many herbal penetration enhancers are included in generally recognized as safe substances list, and they possess low side effects and irritancy in comparison with synthetic chemicals such as solvents and azones or surfactants7.
There are synthetic permeation enhancers and natural permeation enhancers that will help in improving the transdermal permeation of poorly absorbed drugs.8
Dimethyl sulfoxide (DMSO) is a molecule with a long history in pharmaceutics. It is now well established as a penetration enhancer in topical pharmaceutical formulations.9
It promotes permeation by reducing skin resistance to drug molecules or by promotion of drug partitioning from the dosage form.6 However, besides its pharmacological applications, several systemic side-effects from the use of DMSO have been reported, namely nausea, vomiting, dermatologic reactions and toxic effects, particularly on the peripheral nervous system10-12. DMSO penetrates the cell membrane and causes an increase in osmolality both inside and outside the cell, preventing any significant hemolysis due to the formation of an osmotic gradient13. Thus, a natural permeation enhancer will prove to be more effective and beneficial. Aloe vera is among the various natural permeation enhancers. It is an important and traditional medicinal plant belonging to the family Liliaceae. It is indigenous to Africa and Mediterranean countries. It is well known for its succulent leaves and the medicinal and cosmetic properties of the gel obtained from them. Aloe vera has been used externally to treat various skin conditions such as cuts, burns and eczema. It is alleged that sap from Aloe vera eases pain and reduces inflammation. It has antiseptic and antibiotic properties, which make it highly valuable in treating cuts and abrasions. It has also been commonly used to treat first and second degree burns, as well as sunburns and poison oak, poison ivy, and poison sumac infections, and eczema. It can also be used as a hair styling gel and works especially well for curly or fuzzy hair. It is also used for making makeup, moisturizers, soaps, sunscreens, shampoos and lotions14,15. Thus, the objective of this work was to investigate the potential of DMSO and Aloe vera as penetration enhancers for topical delivery of lidocaine.
MATERIALS AND METHODS
Chemicals and reagents
An UV-Visible double beam spectrophotometer JASCO V 630 with 1 cm matched quartz cells was used. Lidocaine was obtained as a gift sample from Ipca Labs Ltd., Ratlam, India. Carbopol 934 (Merck Ltd), benzoic acid (SD Fine Chemical Ltd), Glycerine (Merck Ltd), sodium lauryl sulphate (Supreme Chemicals), glycerine (Supreme Chemicals), triethanolamine and DMSO (SD Fine Chemical Ltd) were procured. Analytical grade chemicals were used for the study. Glass slides having measurement
Plant material
Leaves of Aloe vera were collected from local areas of Kasegaon, District Sangli, (MS), India. The plant material was further identified and authenticated by the Department of Botany, YC College of Science, Karad. The plant material was cleaned thoroughly, dried in shade and stored properly till further use.
Extraction of Aloe vera gel
Spikes and margins were removed carefully before slicing the leaf. The cortex was carefully separated from the parenchyma using a scalpel-shaped knife. Aloe vera gel was extracted by simple drain procedure, where 2-4 leaves of aloe were cut at about ½ inch from the base so as to drain out all the yellow sap material. The sap flows freely with the pressure of the epidermal cells and all other cellular structures above it. Then those leaves were further pressurized with hand till it oozed out clear gel. This procedure was very crude and time consuming as well, but gel obtained by this method was more stable and less degraded than other extraction16,17.
Preparation of lidocaine Gel (with Aloe vera and DMSO)
In the formulation glycerine was used as humectant, benzoic acid was used as a preservative and triethanolamine was employed for adjustment of pH. Carbopol 934 was dissolved in a mixture of glycerin and sodium lauryl sulphate (as surfactant)18 at 80-85°C on a water bath with constant stirring. The mixture was cooled to 40 °C. Aloe vera gel was gradually added to the above mixture with constant stirring to obtain mucilaginous consistency. The stirring was stopped as the gel consistency increased. Lidocaine and benzoic acid was then added and the mixture was stirred. Finally, triethanolamine was added then it was stirred by using propeller for 2 hours at 500 rpm. The entire procedure was performed by addition of DMSO instead of Aloe vera gel. The composition of the formulations is highlighted in (Table 1).
Table 1: Composition of lidocaine gel formulations
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Carbopol 934 | 0.3% | 0.3% | 0.3% | 0.3% | 0.3% | 0.3% |
| Triethanolamine | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Benzoic acid | 0.2% | 0.2% | 0.2% | 0.2% | 0.2% | 0.2% |
| Sodium lauryl sulphate | 2.5% | 2.5% | 2.5% | 2.5% | 2.5% | 2.5% |
| Lidocaine | 2% | 2% | 2% | 2% | 2% | 2% |
| Glycerin | 1.3% | 1.3% | 1.3% | 1.3% | 1.3% | 1.3% |
| Dimethyl sulfoxide | 1% | 2% | 3% | -- | -- | -- |
| Aloe vera | -- | -- | -- | 1% | 2% | 3% |
| Water (to produce 100 gm) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
Evaluation of lidocaine gel
Physical examination
The prepared formulations were inspected visually for their physical appearance, color, texture, phase separation, and homogeneity.
pH
The pH of gel formulations was determined by using digital pH meter (Systronics digital-DI-707). 2.5 g of gel was accurately weighed and dispersed in 25 mL of distilled water and stored for 2 hours and the measurement of pH of each formulation was recorded.
Viscosity measurements
Gels were tested for their rheological characteristics at 25 0C using Brookfield viscometer (DV-III programmable Rheometer). The measurement was made over the whole range of speed settings from 10 rpm to 100 rpm with 30 s between two successive speeds and then in a descending order19,20.
Spreadability
A special apparatus as suggested by Mutimer,7 was designed for determining spreadability of the prepared gel formulations. A bottom glass slide was mounted on fixed base. 2 g of prepared gel formulation was applied on fixed base slide. Then the prepared gel was sandwiched between the top slides having the same dimension of base slide and also provided with a hook. 50 g weight was placed on the top of the two slides for 5min to eject air and to get a uniform film of the gel. Top plate was then subjected to a drag of 20 g weight with the help of a string attached to the hook and the time (in seconds) required by the top slide to cover a distance of 7.5 cm was noted21 .
It is expressed in terms of time in seconds taken by two slides to slip off from the gel and placed in between the slides under the direction of certain load. Lesser the time taken for separation of two slides, better the spreadability. Spreadability was calculated by using the formula:
S = M. L / t.
Where, M is the weight (g) tied to the upper glass slide; L is the length (cm) moved on the glass slide; and t is time to separate the slide (s). In this present experiment, M = 50 g, ‘S’ is recorded.
Centrifugation
Centrifugation test for formulation kept at different storage conditions were performed for 30 days and observed phase separation after centrifugation.
Drug content
Drug content of gel formulations (1 g) was determined by dissolving an accurately weighed quantity of formulation in about 50 mL of pH 6.8 phosphate buffer. The resulting solutions were filtered and subjected to spectrophotometric analysis at λmax 263 nm. If necessary further dilutions were made using the same buffer solution. Drug content was thus calculated22.
In-vitro diffusion study
The diffusion studies were performed by applying 1 g of the gel uniformly to the dialysis membrane. The membrane was mounted between the compartments of Franz diffusion cell. Reservoir compartment was filled with 15 mL of 6.8 pH phosphate buffer. The study was carried out at (37±2) °C and was carried out for 24 h. The sample (1 mL) was withdrawn from reservoir compartment in successive intervals. Each time reservoir compartment was replenished with 1 mL of 6.8 pH phosphate buffer solution to maintain sink condition.
Stability studies
Stability testing of drug product being as a part of drug discovery and ends with the commercial product, to assess the drug and formulation stability, some studies were done. The stability study was carried out for the most satisfactory formulation. The most satisfactory formulation was kept at 0 °C (27±2) °C and (40±2) °C. At the end of 1 month, the samples were analyzed for the drug content and in-vitro diffusion study23.
Kinetic analysis of the release data
In vitro drug release data were studied by using the following equation, as proposed by Korsmeyer24.
Mt = M1 ¼ k tn
Where, Mt/M1 indicated the fractional amount of the drug released from the formulation under study at time t, k refers to the release rate constant and n, indicates the diffusion exponent that states the type of the release mechanism of drug during the release process. Values of n and k were determined by using the linear regression of log (Mt/M1) versus log t.
RESULTS AND DISCUSSION
Table 2: Physicochemical Evaluation data of lidocaine gel
| Formulation Code | pH* | Viscosity* (cps ×103) | Spreadability and consistency* (gm-cm2) | Homogeneity | Drug content*(%) | Diffusion profile* (%) |
|---|---|---|---|---|---|---|
| F1 | 6.76±0.05 | 94±3.46 | 7.33±0.05 | Good | 87.11 ±1.59 | 44.75±1.02 |
| F2 | 6.86±0.05 | 92±2.00 | 5.46±0.20 | Excellent | 89.54±2.09 | 71.14±1.54 |
| F3 | 7.10±0.20 | 90±0.57 | 8.50±0.20 | Excellent | 96.10 ± 0.05 | 84.52±0.82 |
| F4 | 7.0±0.10 | 92±3.05 | 7.50±0.10 | Good | 81.15+0.17 | 52.25±1.50 |
| F5 | 7.0±0.10 | 91±1.15 | 8.30±0.20 | Good | 83.65+0.34 | 63.97±1.79 |
| F6 | 6.93±0.15 | 90±0.57 | 8.23±0.05 | Good | 85.16+0.12 | 79.18±1.10 |
*The results are showed in mean±S.D with three replicates
Table 3: Physicochemical evaluation of the gel formulations (After 30 days)
| Formulation Code | pH* | Viscosity* (cps ×103) | Spreadability and consistency* (gm-cm2) | Homogeneity | Drug content*(%) | Diffusion profile* (%) |
|---|---|---|---|---|---|---|
| F1 | 6.7±0.05 | 94±3.21 | 7.30±0.12 | Good | 87.01 ±0.14 | 45.12±0.97 |
| F2 | 6.3±0.20 | 92±0.57 | 5.40±0.02 | Good | 89.22±1.10 | 70.07±1.66 |
| F3 | 7.2±0.10 | 90±2.08 | 8.53±0.15 | Good | 95.34 ± 1.08 | 82.66±2.00 |
| F4 | 6.7±0.20 | 92±1.52 | 7.50±0.05 | Good | 79.66+0.47 | 50.84±0.65 |
| F5 | 7.0±0.05 | 91±1.15 | 8.33±0.10 | Good | 80.77+1.03 | 62.05±0.81 |
| F6 | 6.9±0.40 | 90±2.51 | 8.35±0.11 | Good | 85.06+0.17 | 79.11±0.95 |
*The results are showed in mean±S.D with three replicates
Table 4: Rheological measurements of prepared gel using DMSO and prepared gel using Aloe vera (After 30 days)
| Shear Rate | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Viscosity in cps ×103 (Mean±S.D) | ||||||
| 10 | 93.92±1.64 | 92.26±2.15 | 90.00±1.25 | 91.52±2.89 | 90.83±2.50 | 89.25±2.00 |
| 20 | 52.63±2.10 | 52.34±2.15 | 51.67±3.73 | 51.37±2.91 | 51.31±2.90 | 50.06±3.54 |
| 30 | 35.73±3.17 | 35.60±2.00 | 34.72±2.64 | 35.74±2.66 | 35.46±3.99 | 34.72±1.50 |
| 40 | 28.53±1.84 | 28.30±3.80 | 27.76±2.38 | 27.49±3.03 | 27.46±3.02 | 26.95±2.52 |
| 50 | 25.40±2.29 | 24.84±1.61 | 24.06±2.80 | 24.71±3.62 | 24.04±2.65 | 23.59±2.21 |
| 60 | 23.45±2.34 | 23.76±2.54 | 22.42±2.26 | 23.18±3.00 | 23.15±2.99 | 22.68±2.50 |
| 70 | 22.35±3.13 | 21.53±2.53 | 21.64±2.67 | 21.40±3.14 | 21.38±2.87 | 20.62±2.22 |
| 100 | 21.36±2.99 | 20.61±2.38 | 19.88±1.52 | 20.39±2.87 | 20.32±2.05 | 19.69±1.72 |
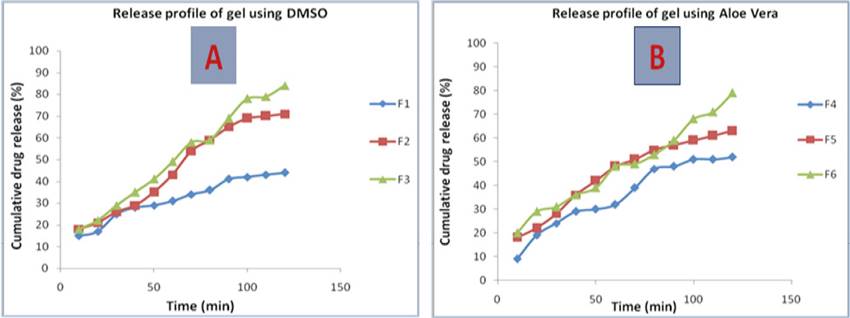
Figure 1: Drug Release profile of A) lidocaine gel containing DMSO B) lidocaine gel containing Aloe vera
Aloe vera (Aloe barbadensis Miller) gel has shown potential to enhance the permeation of certain drug molecules through skin membranes. Aloe vera gel is the viscous, transparent, and colorless mucilaginous gel obtained from the parenchymatous cells in the fresh leaves23,25.
In the present study, six formulations of lidocaine gel were prepared using permeation enhancers namely DMSO and Aloe vera in different concentration viz. (1%, 2% and 3% as highlighted in (Table 1). The prepared formulations were evaluated for physicochemical evaluation.
Physical Examination
The results of the test of physical evaluation revealed that for all the formulations there was no change in color, odor and appearance up to the observation period of 30 days.
The centrifugation test results showed that no phase separation, after centrifugation were found in formulations F1-F6 at 8 ºC and 40 ºC during one month study. All the prepared formulations were found to be colorless with semisolid consistency and characteristic odor. No separation of layer was observed after centrifugation tests. Similar results were observed after 30 days of stability studies for all above said parameters.
pH of the formulations was found to be in the range of 6.8 to 7.1 shown in Table 2. After 30 days the pH of the formulations does not exhibited marked change in the pH values (6.3 to 7.2). The results are shown in (Table 3).Viscosity of the formulations, kept at storage conditions for 30 days, was found to be within the range showed in Table 4. Pseudo-plastic or shear thinning fluids display viscosity reduction while the shear rate increases. Moreover, all the prepared formulations were found to possess good spreadability, consistency and homogeneity.
Drug content
Drug content was found to be in the range of 81.15% to 96.10% in the formulations (Table 2). After one month study the drug content was found to be in the range of 79.66% to 95.34%. (Table 3)
In-vitro diffusion study
In vitro drug release profiles showed that as the concentrations of A. vera gel increased in the formulations, the drug release rate increased substantially (Figure 1) (Figure 2). It was found to be 79.18% for the formulation F6, which comprised of 3% Aloe vera incorporated as a permeation enhancer. Similarly, for formulation F3 that comprised of 3% DMSO as permeation enhancer the drug release was found to be 84.52%. Thus, Aloe vera gel may be considered as an effective permeation enhancer.
Kinetic analysis of the release data
The observed drug release data from the different gel formulations were treated mathematically as per Krosmeyer equation. The obtained results are represented in Table 5. Mathematical treatment revealed that the values of n (release exponent) fall in the range between 0.5 and 1.0 for all the prepared formulations. Thus, it exhibited a non-fickian release pattern governed by a combination of diffusion and chain relation mechanism. Our findings are in accordance with the earlier reported data in British Pharmacopeia, 2007.
Table 5: Kinetic constants (K), diffusional exponents (n) and correlation coefficients (r2) by linear regression of ln (Mt/M1) vs ln t. The values are mean ± SD, n =3
| Formulation Code | n | k | r2 |
|---|---|---|---|
| F1 | 0.7025±0.2257 | 0.8754±0.5647 | 0.9372 |
| F2 | 0.8954±1.2352 | 0.9523±1.0234 | 0.9574 |
| F3 | 0.9215±0.9541 | 0.9635±0.6574 | 0.9593 |
| F4 | 0.6945±0.8451 | 0.9153±0.8971 | 0.9634 |
| F5 | 0.8975±0.7564 | 0.9452±0.7895 | 0.9268 |
| F6 | 0.9025±0.5789 | 0.9532±0.6574 | 0.9183 |
CONCLUSION
Since, the external use of Aloe vera on intact skin is not associated with adverse reactions and is generally regarded as safe, the use of this natural resource as a penetration enhancer may be considered beneficial as compared to other synthetic permeation enhancers. Based on the results of the study it may be concluded that the transdermal gel of lidocaine prepared along with Carbopol 934 by using Aloe vera as a natural penetration enhancer at a concentration of 3% may be used to enhance the penetration for drug molecules across the skin.















