Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
REC: Interventional Cardiology
versión On-line ISSN 2604-7276versión impresa ISSN 2604-7306
REC Interv Cardiol ES vol.5 no.2 Madrid abr./jun. 2023 Epub 18-Mar-2024
https://dx.doi.org/10.24875/recic.m22000353
ORIGINAL ARTICLES
Clinical outcomes of patients undergoing percutaneous coronary intervention treated with colchicine
aDepartment of Cardiology, MedStar Washington/Georgetown University Hospital Center, Washington, D.C., Estados Unidos
bDepartment of Cardiology, Hospital Universitario y Politécnico La Fe, Valencia, España
cCentro de Investigación Biomédica en Red Enfermedades Cardiovaculares (CIBERCV), España
dDepartment of Medicine, MedStar Washington Hospital Center, Washington D.C., Estados Unidos
eDepartment of Medicine, NYU Langone Health, New York, Estados Unidos
fSection of Interventional Cardiology, MedStar Washington Hospital Center, Washington D.C., Estados Unidos
Abbreviations
ACS: |
acute coronary syndrome. |
MI: |
myocardial infarction. |
NSTEMI: |
non-ST-elevation acute myocardial infarction. |
PCI: |
percutaneous coronary intervention. |
RCT: |
randomized controlled trial. |
INTRODUCTION
Despite increasingly effective primary and secondary preventive treatments, coronary artery-related events continue to be the leading cause of morbidity and mortality worldwide.1,2 Lifestyle changes (eg, weight loss, low-salt diet, smoking cessation), medical therapy (eg, anti-hypertensive, lipid-lowering, glucose-lowering, and antithrombotic regimens) in addition to coronary revascularization via percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) constitute the multifaceted approach of this disease. Yet despite the advances made in this multimodality approach, cardiovascular morbidity and mortality remain high.
More recently, the central role played by inflammation in the pathogenesis of coronary artery disease from atherosclerotic plaque formation to acute coronary syndrome (ACS), and PCI itself have gained important recognition. Colchicine, an anti-inflammatory agent indicated for multiple inflammatory conditions including pericarditis, gout, and familial Mediterranean fever, has gained attention as a potential attenuator of atherosclerotic inflammation. Acting via the inhibition of tubulin polymerization and eventually blunting immune cell activation and inflammatory response,3,4 recent evidence suggests a benefit of colchicine in the management of the cardiovascular events of patients with clinical signs of coronary artery disease.5 However, its impact among patients in the peri-PCI period remain controversial.
Recent trials have begun exploring the effects of colchicine in the PCI setting, albeit with mixed results. In the Colchicine-PCI trial of patients with non-ST-segment elevation acute coronary syndrome, the administration of colchicine immediately before and after PCI resulted in lower interleukin-6 and high-sensitivity C-reactive protein (hsCRP) levels at 24 hours, but did not show fewer PCI-related myocardial injuries.6 This trial was followed by COPE-PCI that found that when administered 6-to-24 hours before the PCI, colchicine did in fact reduce PCI-related myocardial injuries in a population of patients with stable angina and non-ST-elevation acute myocardial infarction (NSTEMI).7 Nevertheless, the more recent COVERT-MI trial8 found no difference in infarct size or left ventricular remodeling on the cardiac magnetic resonance imaging in patients treated with colchicine compared to those untreated with this agent.
These individual studies may not provide properly powered analyses, particularly in low-rate events such as strokes, on the impact of colchicine regarding secondary prevention in patients in the peri-PCI period, thus prompting the need for a systematic appraisal and meta-analysis of the quality of evidence and treatment effects on major adverse cardiovascular events.
METHODS
Protocol
The search process of this meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines and is registered with PROSPERO (CRD42021247704). The meta-analysis did not require specific institutional review board approval since it utilized results published in former studies. All relevant information can be found in the trials included. The corresponding author had full access to all the data and final responsibility on the decision to submit the manuscript for publication. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Search strategy
We performed a comprehensive literature search of all published studies retrospective, observational, and randomized controlled trials available on Web of Science, Embase, PubMed, Ovid MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov (inception through August 23, 2021, without language restrictions. Case reports, letters to the editor, reviews, and book chapters were not included in this meta-analysis. The keywords used in the search were ‘colchicine,' ‘coronary artery disease,' ‘coronary heart disease,' ‘angina,' ‘myocardial infarction,' ‘acute myocardial infarction,' ‘myocardial ischemia,' ‘acute coronary syndrome,' ‘ischemic heart disease,' ‘percutaneous coronary intervention,' ‘percutaneous transluminal angioplasty,' ‘percutaneous coronary revascularization,' and ‘myocardial revascularization' including their subheadings, MeSH terms, and all synonyms. References for each of the studies se lected were also screened (the detailed search strategy can be found on the supplementary data). The search process was reported according to the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Selection criteria
Studies were eligible if they included any the following criteria: a) compared the efficacy of colchicine treatment, at any dose and for any duration, to standard medical treatment with or without placebo; b) included populations of patients treated with PCI regardless of the indication; and c) reported, at least, 1 of the following cardiovascular outcomes: all-cause mortality, cardiovascular mortality, myocardial infarction (MI), stroke or urgent coronary revascularization. Study selection was conducted by 2 independent reviewers (C.E. Soria Jiménez, and J. Chang) first by screening titles and abstracts and then by reviewing full texts and their corresponding references. In case of disagreement over eligibility, a third reviewer (H.M. García-García) assessed discrepancy, and decisions were reached by consensus.
Data collection and study endpoints
Data on study characteristics, patient characteristics, and endpoint event rates were independently drawn and organized into a structured dataset by 2 reviewers (C.E. Soria Jiménez, and F. Hayat), and then compared. All discrepancies resulted in the re-evaluation of primary data and involvement of a third reviewer (H.M. García García). Disagreements were resolved by consensus.
Endpoints
The prespecified primary endpoint was all-cause mortality. Secondary clinical endpoints were cardiovascular mortality, MI, stroke, and any revascularization. Each endpoint was assessed according to the definitions reported in the original study protocols (summarized on table 1 of the supplementary data).
Table 1. Characteristics of trials selected
| Trial/Author | Year | Study design | Multicenter | Patients (n) | Population | Colchicine dose and duration | Time elapsed from PCI to start of colchicine | Follow-up |
|---|---|---|---|---|---|---|---|---|
| COVERT-MI8 | 2021 | RCT | Yes | 192 | Adults with a first-time STEMI referred for primary or bailout PCI | 2 mg oral loading dose followed by daily oral 0.5 mg twice daily for 5 days | Loading dose immediately before PCI; if not possible, immediately after PCI | 3 months |
|
| ||||||||
| COPE-PCI7 | 2021 | RCT | No | 75 | Adults with stable angina or NSTEMI undergoing angiography and PCI | 1 mg followed by 0.5 mg 1 h later, 6 hrs to 24 hrs pre-PCI | 6 hrs to 24 hrs before coronary angiogram | 1 day |
|
| ||||||||
| Colchicine-PCI6 | 2020 | RCT | No | 400 | Adults with suspected ischemic heart disease or ACS referred for angiography with possible PCI | 1.2 mg 1 h to 2 h pre-angiography, 0.6 mg 1 h later or immediately after the procedure if rushed for emergency angiography | 1 h to 2 h before coronary angiography | 1 month |
|
| ||||||||
| COPS9 | 2020 | RCT | Yes | 795 | Adults presenting with ACS and evidence of CAD treated with angiography and managed with PCI or medical therapy | 0.5 mg twice daily for 1 month, then 0.5 mg daily for 11 months | Immediately after PCI and randomization | 13.2 months |
|
| ||||||||
| LoDoCo-MI10 | 2019 | RCT | No | 237 | Adults who sustained a type 1 MI within the past 7 days | 0.5 mg daily for 30 days | 1.5 days following the index MI | 1 month |
|
| ||||||||
| Talasaz11 | 2019 | RCT | No | 196 | Adults presenting with STEMI undergoing PCI | NA | NA | 1 month |
|
| ||||||||
| COLCOT I5 | 2019 | RCT | Yes | 4745 | Adults with MI within the past 30 days who had completed some percutaneous revascularization | 0.5 mg once daily for, at least, 2 years | 13.5 days | 42 months |
|
| ||||||||
| Vaidya17 | 2018 | Observational | No | 80 | Adults who presented with ACS < 1 month prior and underwent invasive coronary angiography and revascularization if indicated | 0.5 mg once daily for 1 year | NA (< 1 month from ACS per inclusion criteria) | 12.6 months |
|
| ||||||||
| COLIN12 | 2017 | RCT (Open-label) | No | 44 | Adults admitted for STEMI with occlusion of 1 of the main coronary arteries treated with PCI | 1 mg once daily for 1 month | On the first day of the AMI | 1 month |
|
| ||||||||
| Deftereos 201513 | 2015 | RCT (Pilot) | Yes | 151 | Adults presenting with STEMI of ≤ 12-hour evolution from pain onset treated with PCI | 2 mg loading dose, 0.5 mg twice daily for 5 days | Immediately after completion of diagnostic coronary angiography | 5 days |
|
| ||||||||
| Deftereos 201314 | 2013 | RCT | No | 222 | Adults with diabetes, aged 40-80 treated with PCI with bare metal stent | 0.5 mg twice daily for 6 months | Within 24 hrs of index PCI | 6 months |
|
| ||||||||
| COOL15 | 2012 | RCT | No | 80 | Adults with ACS or acute ischemic stroke | 1 mg once daily for 30 days | Immediately after randomization | 1 month |
|
| ||||||||
| O'Keefe16 | 1992 | RCT | No | 197 | Adults who underwent elective angioplasty (single or multivessel, new or restenosed lesions) for silent, stable or unstable angina; CABG | 0.6 mg twice daily for 6 months | Somewhere between 12 hrs before and 24 hrs after balloon angioplasty | 6 months |
ACS, acute coronary intervention; CABG, coronary artery bypass graft; CAD, coronary artery disease; MI, myocardial infarction; NA, not available; NSTEMI, non-ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST-segment elevation myocardial infarction.
Risk of bias
The risk of bias in each study was assessed using the revised Cochrane Risk of Bias tool (RoB 2.0) for randomized controlled trials (RCTs), and the Risk of Bias in Non-randomized Studies of Interventions assessment Tool from the Cochrane handbook (ROBINS-I) for observational studies. Two investigators (C.E. Soria Jiménez, and J. Sanz Sánchez) independently assessed 5 domains of bias in RCTs: (1) randomization process, (2) deviations from intended procedures, (3) missing outcome data, (4) outcome measurement, and (5) selection of results reported. The same investigators independently assessed 7 domains of bias in observational studies: (1) confounding, (2) selection of participants, (3) classification of procedures, (4) deviations from intended interventions, (5) missing outcome data, (6) outcome measurement, and (7) selection of results reported (table 2 and 3 of the supplementary data).
Table 2. Baseline characteristics of patients from each trial
| Trial/Author | Mean Age | Men (%) | ACS (%) | DES (%) | HTN (%) | DM2 (%) | HLD (%) | Previous MI (%) | Previous PCI (%) | Previous CABG (%) | Underwent PCI (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVERT-MI8 | 60 | 80.3 | 100 | 95.7 | 30.8 | 13.1 | 33.1 | 0 | 0 | 0 | 93 | |
|
| ||||||||||||
| COPE-PCI7 | 64.7 | 71.5 | 58.7 | 97.0 | 54.5 | 22.9 | 63.5 | 17.5 | 16.0 | NA | 100 | |
|
| ||||||||||||
| Colchicine-PCI6 | 66.3 | 93.5 | 49.5 | NA | 91.7 | 57.8 | 88.8 | 25.8 | 37.6 | NA | 100 | |
|
| ||||||||||||
| COPS9 | 59.9 | 79.5 | 100.0 | NA | 50.5 | 19.0 | 46.0 | 15.0 | 13.0 | 4.5 | 88 | |
|
| ||||||||||||
| LoDoCo-MI10 | 61.0 | 77.0 | 100.0 | NA | 47.5 | 22.0 | NA | 15.0 | 11.5 | NA | 90 | |
|
| ||||||||||||
| Talasaz11 | NA | NA | 100.0 | NA | NA | NA | NA | NA | NA | NA | 100 | |
|
| ||||||||||||
| COLCOT I5 | 60.6 | 80.9 | 100.0 | NA | 51.1 | 20.2 | NA | 16.2 | 16.9 | 3.2 | 93 | |
|
| ||||||||||||
| Vaidya17 | 57.4 | 77.5 | 100.0 | NA | 53.8 | 31.3 | 85.0 | 51.3 | 63.8 | NA | 77.5 | |
|
| ||||||||||||
| COLIN12 | 59.9 | 79.4 | 100.0 | NA | 43.4 | 13.7 | 36.5 | NA | 4.6 | 2.4 | 100 | |
|
| ||||||||||||
| Deftereos 201513 | 58.0 | 69.0 | 100.0 | NA | 39.5 | 21.5 | 52.0 | 0.0 | NA | NA | 100 | |
|
| ||||||||||||
| Deftereos 201314 | 63.6 | 65.5 | 31.0 | 0 | 48.5 | 100.0 | NA | NA | NA | NA | 100 | |
|
| ||||||||||||
| COOL15 | 57.2 | 88.8 | 91.3 | NA | 42.5 | 16.3 | 47.5 | 17.5 | 0 | NA | 73 | |
|
| ||||||||||||
| O'Keefe16 | 60.5 | 86.0 | 39.5 | 0 | NA | 12.0 | NA | NA | NA | 25.5 | 100 | |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; DES, drug-eluting stent; DM2, diabetes mellitus type 2; HLD, hyperlipidemia; HTN, hypertension; MI, myocardial infarction; NA, not available; PCI, percutaneous intervention.
Statistical analysis
Odds ratios (OR) and 95% confidence intervals (95%CI) were estimated using the DerSimonian and Laird random-effects model with the estimate of heterogeneity taken from the Mantel-Haenszel method. The presence of heterogeneity among the studies was evaluated using the Cochran Q test referred to chi-square distribution (P ≤ .10 was considered statistically significant) plus the I2 test to assess inconsistencies. Values of 0% indicated no observed heterogeneity, and values ≤ 25%, ≤ 50%, and > 50% indicated low, moderate, and high heterogeneity, respectively. The presence of publication bias was investigated using Harbord test and visual estimation with funnel plots. We conducted a leave-one-out sensitivity analysis for all outcomes by iteratively removing 1 study at a time to confirm that our findings were not driven by any single study. To account for the different follow-up durations across the studies, another sensitivity analysis was conducted using a Poisson regression model with random intervention effects to calculate the means of inverse-variance weighting of trial-specific log stratified incidence rate ratios. Results were shown as incidence rate ratios, which are exponential coefficients of the regression model.
A meta-regression analysis was conducted using the empirical Bayesian method to estimate the between-study variance tau-squared to assess the effect of colchicine dosage, follow-up duration, percentage of patients with ACS, and percentage of those with diabetes mellitus on treatment effects on the primary endpoint.
Two-tailed P values < .05 were considered statistically significant. Statistical analyses were conducted using the Stata software version 13.1 (StataCorp LP, College Station, United States).
RESULTS
Search results
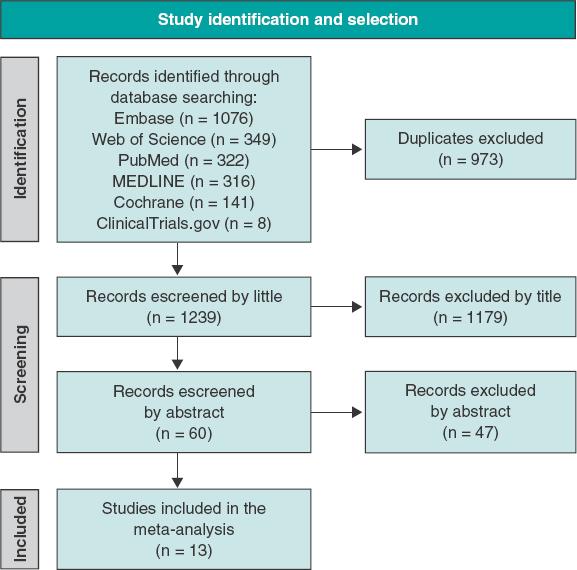
Figure 1 shows the PRISMA study search and selection process. Out of a total of 1239 unique reports, 12 RCTs5-16 and 1 observational study17 were identified and included in this analysis. The corresponding author of the COOL trial15 was contacted regarding data from a number of patients treated with PCI; 58 out of a total of 80 patients evaluated (72.5%) underwent PCI. The study ultimately met the inclusion criteria and was included in our analysis. The main features of the studies included are shown on table 1. Data on the outcomes, mortality, MI, stroke, and urgent revascularization were reported in 12, 9, 5, and 6 trials, respectively. A total of 3741 and 3673 patients treated with and without colchicine were included (for a total of 7414 patients). Time elapsed from the PCI to the start of colchicine went from immediately before PCI to 13.5 days later as shown on table 1.
Baseline characteristics
Main baseline characteristics of the patients included are shown on table 2. Most patients were men with a mean age of 60 years, had ACS, and underwent revascularization with drug-eluting stents.
Publication bias and asymmetry
Funnel-plot distributions of pre-specified outcomes indicate absence of publication bias for all the outcomes (figures 1 to 5 of the supplementary data).
Risk of bias assessment
Table 2 and table 3 of the supplementary data summarize the results of the risk of bias assessment. A total of 11 trials were ranked as trials with a low overall risk of bias, 1 presented some concerns while another one was ranked as a trial with a high overall risk of bias.
Outcomes
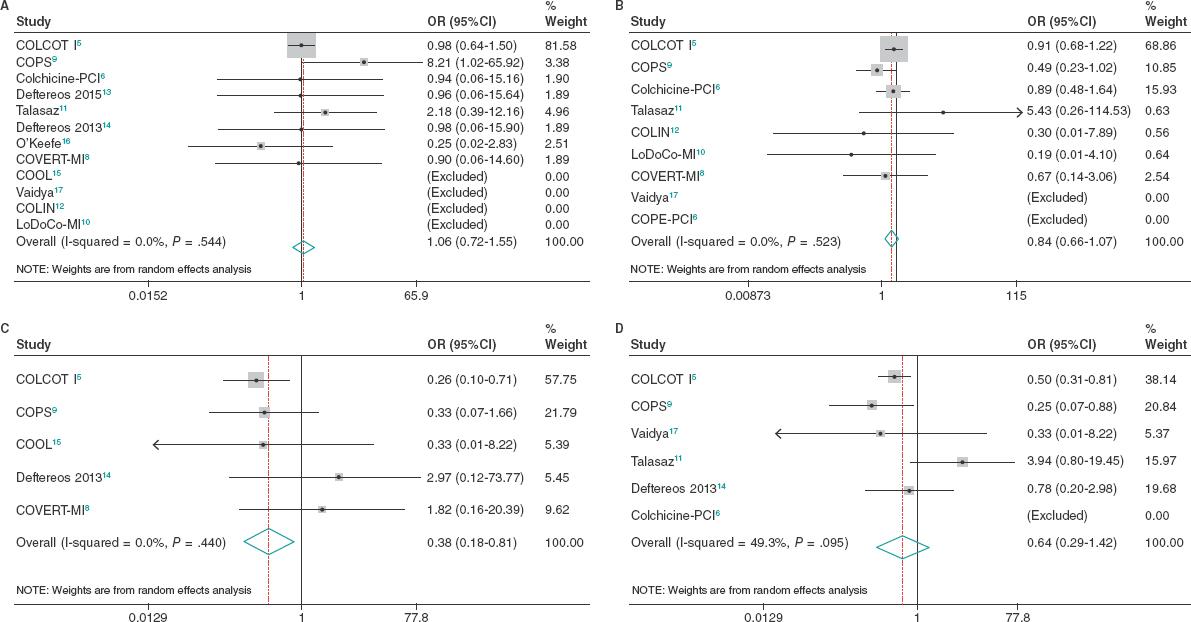
No differences were seen between patients treated with colchicine and those treated without it or placebo regarding all-cause morta- lity (OR, 1.06; 95%CI, 0.72-1.55; I2 = 0%), cardiovascular mortality (OR, 0.98; 95%CI, 0.42-2.28; I2 = 14.2%) or coronary revascularization (OR, 0.64; 95%CI, 0.29-1.42; I2 = 49.3%). However, patients treated with colchicine had a lower risk of stroke (OR, 0.38; 95%CI, 0.18-0.81; I2 = 0%), and a trend towards a lower risk of MI (OR, 0.84; 95%CI, 0.66-1.07; I2 = 0%) (figure 2).
Sensitivity analyses
In the leave-one-out sensitivity analysis, results were consistent with the primary analysis (tables 4 to 8 of the supplementary data). Similarly, in a sensitivity analysis on the use of estimated incidence rate ratios to account for different lengths of follow-up, findings remained unchanged (table 9 of the supplementary data).
When the risk ratios with random-effects models were estimated, findings remained consistent with the main analysis for all endpoints (table 10 of the supplementary data). Random effect meta-regression analyses found no significant impact of colchicine dosage (P = .33), follow-up duration (P = .88), percentage of patients with ACS (P = .37) or percentage of patients with diabetes mellitus (P = .96) on treatment effect regarding the primary endpoint (table 11 of the supplementary data).
DISCUSSION
This meta-analysis included 7414 patients across 12 RCTs and 1 observational study. It showed some clinical benefits on cardiovascular events with the addition of colchicine to standard medical therapy in patients undergoing PCI. Specifically, we found that the addition of colchicine compared to no colchicine or placebo reduced the risk of stroke showing a trend towards a lower risk of MI both with no observed heterogeneity. Additionally, we observed no differences in all-cause mortality, cardiovascular mortality or coronary revascularization. Significantly, colchicine dosage, follow-up duration, percentage of patients with ACS or diabetes mellitus showed no impact on treatment effect (see PRISMA checklist on table 12 of the supplementary data).
Our outcomes regarding all-cause and cardiovascular mortality are consistent with a prior meta-analysis of 5 RCTs conducted by Fu et al.,18 that also found no significant reduction of mortality, MI, serious adverse events, and restenosis. One explanation for the lack of mortality benefit of both trials may be that although mortality rate was generally low and differences were largely not statistically significant in many of these trials, follow-up duration was generally short (< 30 days) in most studies, and it is possible that higher event rates may be seen with longer follow-up data. We should mention that the meta-analysis conducted by Fu et al.18 included 1 RCT of patients treated with CABG, not PCI. It is possible that the inflammatory profiles of this cohort of patients differ from those treated with PCI (eg, multivessel coronary artery disease, longer postoperative recovery, and higher risk of postoperative complications). As a matter of fact, this mixed population may have led to the lack of reduction seen in the overall rate of MI, serious adverse events, and restenosis. Similarly, a prior meta-analysis conducted by Fiolet et al.19 demonstrated that the addition of colchicine to standard medical therapy in patients with acute and chronic coronary syndromes reduced the risk of the primary endpoint significantly (a composite of MI, stroke, and cardiovascular mortality), and the individual endpoint of MI, stroke, and coronary revascularization with no differences whatsoever on all-cause or cardiovascular mortality. Our results demonstrating a lower risk of stroke and a trend towards a lower risk of MI are more consistent with this meta-analysis. A key difference among the different meta-analyses is the population of patients. Fiolet et al.19 included the LoDoCo20 and LoDoCo221 trials whose inclusion criteria were patients with chronic coronary disease and clinical stability for over 6 months. This amounted to > 50% of patients analyzed who were not in the peri-PCI period and likely had a different inflammatory profile at the time of colchicine administration. These 2 trials also had longer follow-ups (36 and 29 months, respectively) potentially allowing for more time to capture outcome differences like MI and urgent revascularization between the different treatment groups. In contrast, our meta-analysis only focused on patients in the peri-PCI as conducted by Fu et al.18 and expanded the total number of studies analyzed to 12 RCTs and 1 observational study. As far as we know, our study is the largest meta-analysis ever conducted to this date to assess the effects of colchicine on the clinical outcomes of patients in the peri-PCI period.
Alkouli et al.22 reported that the adjusted rate of ischemic stroke increased for patients treated with PCI due to ST-segment elevation myocardial infarction (STEMI) (0.6% to 0.96%), NSTEMI (0.5% to 0.6%), and unstable angina or stable ischemic heart disease (UA/SIHD, 0.3% to 0.72%). In turn, in-hospital mortality was higher (23.5% vs 11.0%, 9.5% vs 2.8%, and 11.5% vs 2.4% for STEMI, NSTEMI, and UA/SIHD cohorts, respectively), and post-PCI stroke was associated with a > 2-fold increase in LoS, a > 3-fold increase in non-home discharges, and a > 60% increase in cost. Given the increasing complexity of patients treated as well as the PCI techniques utilized over the past decade, effective preventive strategies and treatments are needed, and herein lies the opportunity for other anti-inflammatory drugs such as colchicine to further mitigate the morbidity and mortality of patients with post-PCI stroke. In the acute phase of MI, activated inflammasomes mount an intense inflammatory response.23 There is also endothelial damage after PCI, which may result in atherosclerotic plaque destabilization with subsequent thromboembolism causing cerebrovascular events.24 Colchicine may play a role preventing stroke by helping stabilize atherosclerotic plaques in patients undergoing PCI, though this effect may not be robust enough to overcome the direct endothelial injury present at the time of PCI.
Colchicine is a widely available drug with known anti-inflammatory properties. Its mechanism of action is yet to be fully elucidated but has been shown to work partly via the inhibition of NLRP3 (nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing protein 3) inflammasome, which ultimately downregulates interleukin-1B and interleukin-6, 2 known inflammatory mediators.23-27 It also causes microtubule disruption and decreased neutrophil activation and extravasation. Since elevated levels of inflammatory biomarkers are an independent predictor of major adverse cardiovascular events28-31 our results show that colchicine joining the current medical therapy is a potential addition to further attenuate inflammation regarding the secondary prevention of cardiovascular disease in patients undergoing PCI.
Some limitations of our study include the use of aggregate study-level data as opposed to patient-level data. While this limits subgroup analyses, the overall conclusions would remain the same. There was also a small percentage of patients in each of the studies analyzed who did not undergo PCI, which poses some limitations on the overall effects on a PCI population. However, in all studies, the vast majority of patients eventually underwent this procedure. Similarly, the LoDoCo221 trial enrolled patients who underwent PCI but was ultimately excluded from this analysis as patients required a period of clinical stability 6 months after PCI before starting colchicine therapy. A 6-month gap from PCI to colchicine initiation did not fit in with our period of interest (the peri-PCI period). The study conducted by O'keefe16 was completed in an era of balloon angioplasty, and colchicine treatment in this setting may not be comparable to patients who underwent PCI in the era of statins, modern stents, and antiplatelet agents. Additionally, most patients from our study underwent PCI due to the presentation of ACS, yet there were other clinical presentations including stable ischemic heart disease and unstable angina, and yet others that specifically excluded patients with acute MI. Given the different clinical status at presentation for PCI, it's likely that the inflammatory profile of these different populations of patients also varied resulting in different clinical outcomes. Nevertheless, despite variation in the inclusion and exclusion criteria, outcome definitions, and colchicine dose and duration, this did not introduce heterogeneity into our results.
CONCLUSIONS
In patients undergoing PCI, the addition of colchicine to optimal medical therapy resulted in a significant reduction of strokes, and a trend towards a lower risk of MI. However, this did not result in lower all-cause and cardiovascular mortality rates, and urgent revascularization.
AUTHORS' CONTRIBUTIONS
M.B. Levine was involved in data curation and research. F. Hayat, and J. Chang were involved in data curation and research, as well as in drafting, editing, and reviewing the early draft of the manuscript. C.E. Soria Jiménez, J. Sanz Sánchez, and H. García-García were involved in project conceptualization, data curation, formal data analysis and investigation, methodology, project administration, resources, validation, visualization, as well as drafting, editing, and reviewing all manuscript drafts and its final version.
WHAT IS KNOWN ABOUT THE TOPIC?
Inflammation plays a central role in the pathogenesis of coronary artery disease, and it's involved in percutaneous coronary interventions. Colchicine is a powerful anti-inflammatory drug. Its effect, however, attenuating peri-PCI inflammation remains unknown.
WHAT DOES THIS STUDY ADD?
REFERENCES
1. Fox KA, Poole-Wilson P, Clayton TC, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome:the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366:914-920. [ Links ]
2. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [ Links ]
3. Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart:pushing the envelope. J Am Coll Cardiol. 2013;62:1817-1825. [ Links ]
4. Nidorf SM, Eikelboom JW, Thompson PL. Colchicine for secondary prevention of cardiovascular disease. Curr Atheroscler Rep. 2014;16:391. [ Links ]
5. Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505. [ Links ]
6. Shah B, Pillinger M, Zhong H, et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention:COLCHICINE-PCI Randomized Trial. Circ Cardiovasc Interv. 2020;13:e008717. [ Links ]
7. Cole J, Htun N, Lew R, Freilich M, Quinn S, Layland J. Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention:The COPE-PCI Pilot Trial. Circ Cardiovasc Interv. 2021;14:e009992. [ Links ]
8. Mewton N, Roubille F, Bresson D, et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation. 2021;144:859-869. [ Links ]
9. Tong DC, Quinn S, Nasis A, et al. Colchicine in Patients With Acute Coronary Syndrome:The Australian COPS Randomized Clinical Trial. Circulation. 2020;142:1890-1900. [ Links ]
10. Hennessy T, Soh L, Bowman M, et al. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study:A pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J. 2019;215:62-69. [ Links ]
11. Talasaz AH, Jenab Y, Hosseini SH. P461. Colchicine before percutaneous coronary intervention in acute myocardial infarction. Eur Heart J.2019(40):Suppl 1;ehz745.0994. [ Links ]
12. Akodad M, Lattuca B, Nagot N, et al. COLIN trial:Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. 2017;110:395-402. [ Links ]
13. Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction:A Pilot Study. Circulation. 2015;132:1395-1403. [ Links ]
14. Deftereos S, Giannopoulos G, Raisakis K, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol. 2013;61:1679-1685. [ Links ]
15. Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke:a pilot randomized controlled trial. J Thromb Thrombolysis. 2012;33:88-94. [ Links ]
16. O'Keefe JH Jr, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol. 1992;19:1597-1600. [ Links ]
17. Vaidya K, Arnott C, Martínez GJ, et al. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome:A CT Coronary Angiography Study. JACC Cardiovasc Imaging. 2018;11:305-316. [ Links ]
18. Fu C, Wang B. Colchicine administration for percutaneous coronary intervention:A meta-analysis of randomized controlled trials. Am J Emerg Med. 2021;46:121-125. [ Links ]
19. Fiolet A, Opstal T, Mosterd A, et al. Efficacy and Safety of Low-Dose Colchicine in Patients with Coronary Disease:A Systematic Review and Meta-Analysis of Randomized Trials. Eur Heart J. 2021;00:1-11. [ Links ]
20. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. [ Links ]
21. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383(19):1838-1847. [ Links ]
22. Alkhouli M, Alqahtani F, Tarabishy A, Sandhu G, Rihal CS. Incidence, Predictors, and Outcomes of Acute Ischemic Stroke Following Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12:1497-1506. [ Links ]
23. Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation.2017;e12305. [ Links ]
24. de Winter RJ, Heyde GS, Koch KT, et al. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J. 2002;23:960-966. [ Links ]
25. Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages:a novel link between cholesterol metabolism and inflammation. PLoS One.2010;5:e11765. [ Links ]
26. Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E. Technical advance:Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94:1091-1096. [ Links ]
27. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-241. [ Links ]
28. Buffon A, Liuzzo G, Biasucci LM, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512-1521. [ Links ]
29. Kwaijtaal M, van Diest R, Bär FW, et al. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis. 2005;182:341-348. [ Links ]
30. Patti G, Di Sciascio G, D'Ambrosio A, Dicuonzo G, Abbate A, Dobrina A. Prognostic value of interleukin-1 receptor antagonist in patients undergoing percutaneous coronary intervention. Am J Cardiol.2002;89:372-376. [ Links ]
31. Walter DH, Fichtlscherer S, Sellwig M, Auch-Schwelk W, Schächinger V, Zeiher AM. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol. 2001;37:839-846. [ Links ]
Received: September 09, 2022; Accepted: November 04, 2022











 texto en
texto en