Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Angiología
versión On-line ISSN 1695-2987versión impresa ISSN 0003-3170
Angiología vol.75 no.5 Madrid sep./oct. 2023 Epub 11-Dic-2023
https://dx.doi.org/10.20960/angiologia.00543
Special Articles
Epidemiological studies or how should we design our research? (part one)
1Angiology, Vascular and Endovascular Surgery. Hospital Universitario Clínico San Carlos. Madrid, Spain
Methodological knowledge is essential to implement our research projects and epidemiological designs. Adequate design will allow us to adequately communicate the results of our clinical experience.
There are different types of design depending on multiple factors such as the active participation of the investigator, the randomization of patients or the composition of the control group. It is essential to be familiar with them in order to know which is the most appropriate to start our journey as researchers.
In this paper we show the characteristics and terminology with which we refer to the different epidemiological designs and we will present some of these designs, which we will finish in future papers.
Keywords: Epidemiological studies; Design; Research
INTRODUCTION
Whenever we think about starting a research project, we face the situation of how to outline the best possible study. And the most important thing of all: which should the optimal study be for the question that we, as researchers, have just posed?
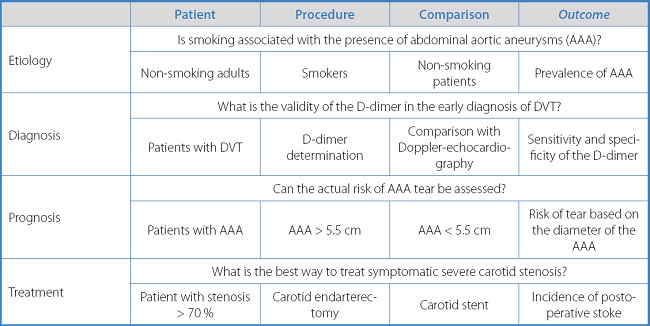
This question that the researcher asks should ideally orbit around 4 elements: patient, intervention, comparison, and outcome.
This question is typically framed within what is referred to as “PICO.” Although the components “intervention” and “comparison” suggest the experimental nature of clinical trials and may seem to restrict the system to issues regarding treatment, it can also be used for questions on etiology, diagnosis, or prognosis, as shown in table I.
This question formulation technique reveals the need researchers have of a structured approach regarding the initiation of such questions and the appropriate studies, on a routine basis, to provide answers to these questions (Table II).
How many times have we heard that a clinical trial is “the best”? Well, it depends. We can hardly conduct a clinical trial to study the association between smoking and AAAs. That would involve an absurdity: randomly force the study participants to smoke and others not to, to see if one group develops more AAAs than the other. Ethical considerations logically prevent these absurdities from happening, which underscores the importance of having a clear understanding of what we want to study to choose the most suitable trial to do so.
The objective I propose is to analyze together the most widely used studies, their characteristics, strengths, and weaknesses. But before doing so, I first suggest to understand the overall characteristics that will allow us to do better individual interpretations of each study.
OBSERVATIONAL VS EXPERIMENTAL TRIALS
An observational design is a study in which the researcher merely “observes.” The study participants decide what they are exposed to, but the researcher only chooses the association he/she wants to analyze without introducing the study factor to the participants or deciding who will receive such factor.
Unlike observational trials, experimental trials are characterized by the researcher's active involvement. On the one hand, the researcher introduces the study factor. On the other hand, he/she decides what participants will receive, or not, the study factor.
Let's consider an example of both situations. Imagine we want to conduct a study to see if atorvastatin reduces cholesterol more than simvastatin does. Firstly, the researcher has introduced the treatments that the subjects will receive. Secondly, the researcher will play a decisive role in determining who will receive either one of the two treatments.
Regarding how the study factor is assigned, it can be randomized, meaning determined by chance, which minimizes the likelihood of biases, or non-randomized (here chance doesn't play a role).
ANALYTICAL VS DESCRIPTIVE TRIALS
A descriptive trial is one that is limited to defining or describing an event seen in a group of participants. There is no option for comparison because there's only one group or cohort under study. For instance, the frequency of smoking in our patients with AAA.
In contrast, analytical trials allow us to “analyze” relationships between risk factors and diseases because there are 2 groups or cohorts. For example, when we're comparing if smoking is more common in patients with AAA than in healthy individuals, what we're actually doing is analyzing the smoking-AAA association, which can be quantified using measures of association such as relative risk or odds ratios.
Based on the parameters discussed above, we can create the following outline of the most common type of clinical trials conducted, as shown in figure 1.
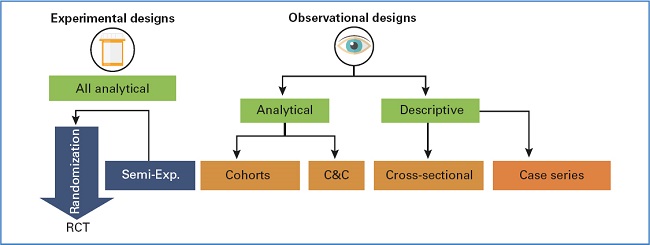
Figure 1. Types of trials. C&C: cases and controls; RCT: randomized clinical trial; Semi-Exp.: semi-experimental.
Regarding the outline shown in figure 1, and although it holds more didactic than theoretical importance, 3 points should be summarized here:
It arranges the types of clinical trials based on their financial burden: cost associated with RCT > cohorts > case-control > cross-sectional.
It ranks the designs based on the causal evidence they provide: RCT > cohorts > case-control > cross-sectional.
It shows the number of biases (systematic errors) inherent to each and every trial: RCT < cohorts < case-control < cross-sectional.
In this article, we will be analyzing some of the most widely used observational designs to date, sparing experimental trials—basically randomized clinical trials—for a different occasion.
OBSERVATIONAL TRIALS
Descriptive observational trials
The objective of descriptive trials is double. On one hand, they aim to describe the characteristics and frequency of a health issue based on personal characteristics (age, sex, marital status, etc.), location (geographical area, etc.), and the time of occurrence of the problem and its trends. Additionally, these studies are the foundation for analytical trials.
Cross-sectional trials
Cross-sectional or prevalence trials analyze the relationship between a disease and certain variables at a specific point in time. They seek to identify a potential link between a risk factor (RF) and a disease, which will later need to be confirmed in analytical trials (2). A good example of this type of trials is the registry from our own society showing the activity developed in our services at a given point in time.
Analyzing data in cross-sectional trials is relatively easy. The association between the prevalence of a disease and the prevalence of exposure to it are studied. The strategy to compare the prevalence between exposed and unexposed groups is similar to that found in other designs. However, here we'll be using the prevalence ratio (prevalence of disease in exposed participants/prevalence of disease in unexposed participants).
The following advantages of prevalence trials are worth mentioning:
- The cross-sectional design repeated over time allows visualization and assessment of trends.
- They facilitate hypothesis generation on factors associated with multiple conditions, particularly useful for the study of common chronic diseases.
- Their cost is low and they're easily reproducible.
- They are less sensitive to memory bias and follow-up losses.
- They typically don't create ethical issues.
These are the 4 most important limitations of cross-sectional or prevalence designs:
They are not suitable for rare diseases because they don't show the mechanism triggering the disease.
They're based on prevalent cases that may not be representative of the routine clinical practice. For instance, prevalent cases of a certain disease are those with higher survival rates.
Because the presence or absence of exposure and disease are determined simultaneously at a given point in time, on many occasions, it won't be possible to establish whether exposure precedes or results from the disease.
They are highly sensitive to non-response bias (a type of selection bias), which thus leading to under- or overestimate the prevalence of the disease analyzed.
CASE REPORT SERIES
Case reports and case series are a significant portion of the scientific output in conferences organized by multiple scientific societies.
They are defined as a type of publication that reports on a series of cases that share common characteristics that makes them groupable such as being syndromic, etiological, anatomical, histological, physiological, genetic, molecular, treatment-related, sharing treatment-related adverse events, or characteristics from a supplementary trial.
An example of this kind of study is the one published by Díaz Cruz et al. (3) on their experience with IMA embolization during EVAR.
Although these designs are very useful to formulate hypotheses, they are not good to assess or test the presence of a statistical association. The presence of an association can be a random finding. The most important limitation of these studies is, ultimately, lacking a control group.
ECOLOGICAL TRIALS
This type of descriptive, observational trial is unique in that the unit of measurement is not the individual, but the collective. As we will discuss below, these trials have some issues but also many advantages, as they allow the study of variables hard to analyze individually. For example, are low atmospheric pressures associated with a higher incidence of AAA tear? Is environmental pollution associated with a higher risk of cardiovascular diseases?
These are trials in which the unit of analysis is groups of individuals, not individuals themselves (such as school classes, cities, regions, etc.). The main characteristic of this type of trial is that information on exposure or the event itself is available for the entire cluster. However, individual information for each member of the group is unknown.
Ecological trials have a wide range of advantages and limitations. These are some of the former:
- Being cost-effective and easy to conduct.
- Being useful as a starting point to generate new hypotheses that need confirmation through analytical approaches. In many cases, exposure cannot be measured individually (eg, pollution). Ecological trials are the best alternative in these cases.
- To assess the effectiveness of certain public health interventions, global rather than individual outcomes can provide more information.
However, these advantages are no stranger to limitations:
- These trials are not suitable to make causal inferences, that is, to establish causality relationships.
- It is not always possible to control for possible confounding factors. Since they depend on secondary data, these trials are constrained by the quality and the availability of information.
- The primary limitation of ecological trials is the ecological fallacy. It is defined as the “extrapolation to the individual level of relative risks estimated for an entire population, without considering that biases may exist that are absent in individual-level risk estimates.”
In upcoming installments, we will explore different types of analytical trials, both observational and experimental.
REFERENCES
1. Martín-Conejero A. Metodología Básica de la Investigación Clínica. Madrid: CTO Editorial; 2019. [ Links ]
2. Torres Blanco Á, Iborra Ortega E, Altable García M. Registro de actividades de la Sociedad Española de Angiología y Cirugía Vascular, año 2018. Angiología 2020;72(3):145-59. [ Links ]
3. Díaz Cruz J, González García A, Arízaga Idobro V, Baeza Bermejillo C, Arribas Díaz A, Aparicio Martínez C. Experiencia inicial en nuestro centro con la embolización preventiva de la arteria mesentérica inferior en el tratamiento endovascular de los aneurismas de aorta abdominal. Angiología 2023;75(4):212-7. DOI: 10.20960/angiologia.00468 [ Links ]
Received: June 22, 2023; Accepted: June 22, 2023











 texto en
texto en