Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos Españoles de Urología (Ed. impresa)
versión impresa ISSN 0004-0614
Arch. Esp. Urol. vol.62 no.3 abr. 2009
Clinical presentation of renal cell carcinoma in renal transplant
Presentación clínica del carcinoma de células renales en el trasplante renal
Raquel González-López, Gonzalo Bueno-Serrano, Javier Mayor-De Castro, José Julián Vázquez-Escuderos, Víctor Díez-Nicolás, Roberto Marcén Letosa, Julio Pascual Santos and Francisco Javier Burgos Revilla.
Urology Section. Nephrology Section. University of Alcalá de Henares. Ramón y Cajal Hospital. Madrid. Spain.
SUMMARY
Objectives: To analyze the clinical presentation and therapeutic response of renal cell carcinoma (RCC) of the renal graft.
Methods: Analysis of the cases described in our centre and review of current literature.
Results: RCC has a higher incidence in transplant patients, affecting the graft in less than 10% of the cases. Detection is usually a casual event during follow-up due to the absence of innervation, although its presentation may be as an acute abdomen in case of breakage of the graft. Conventional treatment consists of transplant nephrectomy, but partial nephrectomy has been performed in recent years with good results. The modification of immunosuppression is a routine measure after treatment.
Conclusions: The incidence of RCC after renal transplants in our series is 0.7%, of which 22% are originated in the graft. The clinical presentation of the primitive RCC of the graft is variable. Partial nephrectomy is technically feasible and oncologically safe in the treatment of RCC of the renal graft.
Key words: Renal cell carcinoma. Kidney transplantation. Immunosuppression. Clinical presentation. Transplantectomy. Partial nephrectomy.
RESUMEN
Objetivo: Analizar la presentación clínica y la actitud terapéutica ante la afectación del injerto por un Carcinoma de células renales (CCR).
Métodos: Análisis de los casos descritos en nuestro Centro y revisión de la literatura actual.
Resultados: El CCR presenta una incidencia superior en los pacientes trasplantados, afectando en menos del 10% al injerto. La ausencia de inervación hace que habitualmente sea un hallazgo casual durante el seguimiento, aunque su presentación puede llegar a ser como un abdomen agudo en caso de rotura del injerto. El tratamiento convencional es la trasplantectomía, realizándose en los últimos años la nefrectomía parcial con buenos resultados. La modificación de la inmunosupresión es una medida habitual tras el tratamiento.
Conclusiones: La incidencia de CCR post-TR en nuestra serie es del 0,7%, originándose el 22% de los mismos en el injerto. La presentación clínica del CCR primitivo del injerto es variable. La nefrectomía parcial es técnicamente posible y oncológicamente segura en el tratamiento del CCR del injerto renal.
Palabras clave: Carcinoma de células renales. Trasplante renal. Inmunosupresión. Presentación clínica. Trasplantectomía. Nefrectomía parcial.
Introducción
The occurrence of a renal cell carcinoma (RCC) in the renal graft is extremely rare. On the other hand, the incidence of RCC in transplant patients is higher than among the general population (4.8% vs. 2.3%). From these tumours, less than 10% affect the graft and their clinical presentation can be extremely diverse, being the incidental detection during follow-up the most common way. Immunosuppression (IS) is one of the most important factors associated with the appearance of tumours in these patients. In fact, the current tendency is that IS is modified after diagnosis and treatment.
Objective
To analyse the clinical presentation and therapeutic response with regard to the graft affection by a renal cell carcinoma (RCC), taking into account the affection degree, presentation and prognosis of each patient. To analyse the current situation of RCC in renal transplants.
Materials and methods
A total of 1,250 renal transplants (RTs) were carried out between 1978 and 2008. Only 9 receivers developed an RCC, 7 of which were situated in the native kidney and 2 in the graft. One of the RCCs of the native kidney presented synchronous and multiple metastatic affection of the graft. The clinical presentation, therapeutic response and evolution of the 3 patients with graft affection by RCC were studied. After an extensive literature review, the most relevant aspects of RCC in connection with RTs are discussed.
Results
Case study 1
Male, 67 years old, with personal history of arterial hypertension, dyslipemia, obstructive hypertrophic myocardiopathy and obstructive sleep apnea syndrome. Chronic renal failure due to nephroangiosclerosis, with transplant in the right iliac fossa in 1998, without incidences. The patient presents a normally functioning graft (serum creatinine: 2.4 mg/dL basal) and receives treatment with Prednisone and Ciclosporine A (CsA). In 2006, a disease is detected in the lower lip, which is removed, with the anatomopathological result of microinfiltrative epidermoid carcinoma. A modification of the immunosuppression is performed, substituting CsA by Tacrolimus.
In May 2007, a routine ultrasound scan detected a solid mass in prior cortical, superior middle area, with a larger diameter of approximately 3 cm. The patient is asymptomatic and with preserved graft function. Confirmation TC is carried out, where a focal and solid disease is detected in the upper middle area of the anterior surface of the graft (Figure 1). After puncture-aspiration, it is confirmed that the disease corresponds to renal cell carcinoma and it is decided to carry out conservative nephron surgery in view of the good functioning of the graft and the favourable characteristics of the disease. Under cold ischemia and with intraoperative ultrasound control, partial nephrectomy with intracapsular approach was performed, sending the tumour site for intraoperative pathological analysis, which confirms the negativity of the surgical margins. The definitive result of the analysis confirmed a renal cell carcinoma, stage pT1 G1, completely removed (Figure 2).
The evolution of the patient is satisfactory, presenting a discrete elevation of creatinine up to 4.2 mg/dl with recovery of the basal values on the fifth day after operation. Currently, after a follow-up of 14 months, the patient is asymptomatic and no data of tumour recurrence or remote metastasis exist. The graft function is stable, with creatinine levels of 2.3 mg/dl. Prior immunosuppression was maintained with Prednisone and Sirolimus was added.
Case study 2 (1)
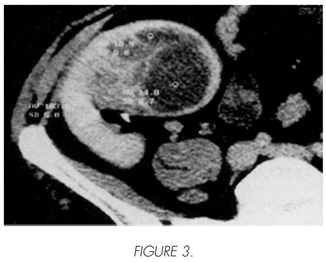
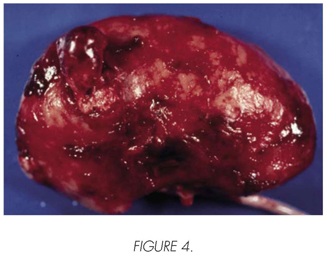
Male, 51 years old, transplanted in 1986 by mesangiocapillary glomerulonephritis type II, with chronic graft dysfunction due to recurrence of his kidney disease, which requires the resumption of the haemodialysis is 1996. A decrease of his immunosuppression took place with CsA and adding Azathioprine, without results. 15 days after the start of the haemodialysis, he presents an acute pain on the graft with progressive haemodynamic instability and refractory to the medical treatment. In the urgent ultrasound scan, a subcapsular and intraparenchymatous haematoma was detected in the graft, confirmed by means of TC (Figure 3). Transplantectomy was carried out a few hours later. During the operation, a solid disease was observed in the upper end of the graft, with a diameter of approximately 4 cm and clear signs of spontaneous rupture and bleeding (Figure 4). Moreover, multiple other small-size solid and cystic diseases were detected on the graft surface. The anatomopathological analysis revealed a multi-centre CCR, within an acquired cystic kidney disease of the graft. The patient resumed the haemodialysis and is now free of disease and asymptomatic after a follow-up of 12 years.
Case study 3 (2)
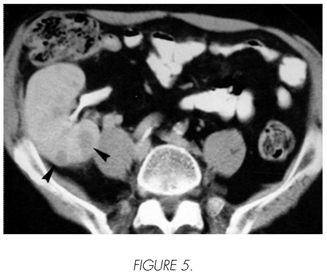
Male, 62 years old, transplanted in 1989 by IgA nephropathy and under immunosuppressive treatment with CsA and Prednisone. Six years after the transplant, he presents monosymptomatic right back pain. In the native right kidney, multiple cysts (ARCD was developed two years after the start of the haemodialysis) and solid renal mass of 7 cm in the lower end are detected with ultrasonography. In the TC, the disease was confirmed and multiple solid diseases were detected in the renal graft, as well as other metastatic diseases in the liver, subcutaneous implants and retroperitoneal adenopathies (Figure 5). The puncture-aspiration with fine needle confirmed the presence of RCC in all diseases. A conservative treatment was started with analgesia and immunosuppression reduction. Three weeks after diagnosis the patient dies.
Discussion
Renal cell carcinoma (RCC) is the most frequent malignant kidney tumour. Its incidence in transplanted patients is slightly higher than in the general population (4.8 vs. 2.3%) (3). At the moment, the cause of this increase has not been established yet, but it could be related to the immunosuppression that the transplanted patients receive. Most of these tumours are situated in the native kidneys, whereas less than 10% affect the graft (4). According to our experience, the incidence of RCC is of 0.66%, affecting the graft in 22%. The average interval of appearance after transplantation cited in literature is 3.5 years (5), although cases in grafts have been described of up to 26 years after transplantation (6). The estimated growth of these tumours is between 0.5 and 1 cm per year (7), identical to the tumours appearing in native kidneys and in patients without transplants. In general, they present a lower aggressiveness in transplanted patients, independently of their location (8), except for the papillary tumour RCC, in which an increased aggressiveness is reported for these patients. Moreover, the papillary tumours are more frequent in the transplanted population than in the general population and are usually associated to the acquired renal cystic disease (ARCD) of the native kidneys (7,9).
The absence of the graft innervation provokes that most patients are asymptomatic to the diagnosis, which is incidental in most cases and which is carried out during the periodic follow-up to which the transplanted patients are subjected. The regular follow-up, in addition to an analytical study of the renal function, usually consists of an image test, generally an annual ultrasound scan (9-12). This technique allows the performance of an initial diagnosis, whereas the images are confirmed by TC afterwards, in order to enable the simultaneous staging. Some authors are inclined to carry out MRI instead of TC, since this technique provides a better visualisation of the tissues and the locoregional extension without nephrotoxic effect of the contrast, also preferring it during follow-up (10, 13-17). However, the availability of this technique and its lower sensitivity with regard to the detection of masses below 3 cm (60%) (10) makes it more common to perform TC.
A number of authors plead for the performance of fine needle puncture-aspiration to confirm the disease type (6, 16-19) as in the first case of the present series, whereas others prefer not to use this technique, considering it hazardous for the graft due to the bleeding risks and the theoretical tumour seeding, circumstances that did never take place in our centre. Due to the high number of false negatives that are produced, they also consider it a low-profitable technique in theses cases (4,10), preferring the anatomopathological confirmation of the piece. In our opinion, this gives rise to the overtreatment of benign diseases that could be diagnosed by means of fine needle puncture-aspiration. The DNA analysis of the surgical piece can indicate the origin of the tumour (10,20-21), since, even though it usually is a primary tumour of the graft, it can also be a metastasis of an RCC situated in the native kidney. It is also possible to determine the origin by means of analysis with fluorescence in situ hybridization (FISH) of the biopsy material (22).
The increase of tumour incidence in the transplanted patients has been related to the immunosuppressive therapy that these patients receiver, among other factors. The tumour development has been demonstrated after the start of the immunosuppression after transplantation, with a high incidence of cutaneous (28-40%) and lymphoid tumours (11-27%) (5,7,23). Kunikata et al (12) carried out a study in the Japanese population, analysing the incidence of tumours in transplanted patients and in dialysis, detecting the existence of an increased incidence of gastric carcinoma and RCC (most frequent tumours in this ethnic group). They also observe a relation between the administration of CsA and the development of RCC in native kidneys in transplanted males.
Some authors have reported an increase of the tumour aggressiveness and a higher possibility of dissemination in transplanted patients due to the immunosuppression (10), preferring therefore to modify the immunosuppressive patterns. The immunosuppressive adjustment is carried out according to the seriousness of the tumours, being the most common procedure its modification in the cutaneous carcinomas (7).
The withdrawal of the CsA is recommended, usually through its substitution by Sirolimus, which is shown to be a strong inhibitor of tumour growth, acting as a protective agent against RCC progression (21,24). It should be kept in mind that RCC is a tumour acting as an inducer of the immune response (21); for that reason, the effect of immunosuppressive agents on RCC is unquestionable. Although normally the transplant is a contraindication for the administration of immunotherapy, several cases are described in literature of its application on HCV infection in patients with renal transplants, leading to good results and without rejection phenomena (7,25). However, studies in this field should be continued.
On the other hand, a number of authors defend the maintenance of the immunosuppressive schemes without modifications, since this will lead to a decrease of the rejection risk and since complications arising from the use of certain immunosuppressors such as Sirolimus will be avoided (11,21,26). Other authors maintain the treatment due to the fact that they have not found a clear relation between immunosuppression and tumours. This way, in a study carried out on 373 transplanted patients, Moudoni et al. (7) have not detected a correlation between the appearance of de novo carcinoma in native kidneys and the age of the receiver, the time of permanence in haemodialysis, the post-RT time or the immunosuppressive pattern. In fact, these authors did not modify the immunosuppression in theses cases.
At present, no consensus exists on the attitude to adopt, although the current tendency is to modify the immunosuppressive treatment, with withdrawal or minimisation of the anticalcineurinic or inclusion of an inhibitor of the mTOR (Sirolimus or Everolimus) in the immunosuppressive pattern. The three patients included in this series were under treatment with CsA, which was suspended in the last two and replaced by Sirolimus in the first.
Conclusions
The incidence of RCC after renal transplants in our series is 0.7%, of which 22% are originated in the graft. In some occasions, these can be affected by metastasis of a primitive RCC of the native kidney. The clinical presentation of the primitive RCC of the graft is variable: from incidental in an asymptomatic patient to an acute abdomen due to graft breakage. The partial nephrectomy is technically feasible and oncologically safe in the RCC treatment of the renal graft.
 Correspondence:
Correspondence:
Raquel González López
Pza Ondarreta, 4 8oC
28923 Alcorcón. Madrid. (Spain).
rakelgon@hotmail.com
Accepted for publication: October 7th, 2008.
References and recomended readings (*of special interest, **of outstanding interest)
1. Fernández-Juárez G, Pascual J, Burgos FJ, Mampaso F, Cano T, Liaño F, et al. Late rupture of the renal graft: not always graft rejection. Nephron Dial Transplant. 1998; 13: 496-498. [ Links ]
2. Gómez García I, FJ Burgos Revilla, E Sanz Mayayo, S Conde Someso, C Quicios Dorado, J Pascual, et al. Metástasis en injerto renal de adeno-carcinoma renal primario. Actas Urol Esp. 2004; 28(6):458-461. [ Links ]
**3. Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transplant 1998; 147-158. [ Links ]
4. Penn I. Primary kidney tumors before and after transplantation. Transplantation 1995; 59: 480-485. [ Links ]
**5. Penn I. Cancers in renal transplant recipients. Adv Ren Replace Ther. 2000; 7: 147-156. [ Links ]
**6. Charboneau JW, O'Loughlin MT, Milliner DS, Engen DE. Sonographically guided percutaneous radio frequency ablation of a renal cell carcinoma in a transplanted kidney. J Ultrasound Med. 2002; 21: 1299-1302. [ Links ]
*7. Moudoni SM, Lakmichi A, Tligui M, Rafii A, Tchala K, Haab F, et al. Renal cell carcinoma of native kidney in renal transplant recipients. BJU Int. 2006; 98: 298-302. [ Links ]
8. Ghasemian SR, Guleria AS, Light JA, Sasaki TM. Multicentric renal cell carcinoma in a transplanted kidney. Transplantation. 1997; 64(8): 1205-1206. [ Links ]
**9. Burgos FJ, Marcén Letosa R, Pascual Santos J, Gómez García I, Sanz Mayayo E, Gómez Dosantos V, et al. Carcinoma renal y trasplante renal. Urol Integr Invest 2005; 10(1): 46-54. [ Links ]
10. Moudouni SM, Tligui M, Doublet JD, Haab F, Gattegno B, Thibault P. Nephron-sparing surgery for de novo renal cell carcinoma in allograft kidneys. Transplantation. 2005; 80(6): 865-867. [ Links ]
*11. Ribal MJ, Rodríguez F, Musquera M, Segarra J, Guirado L, Villavicencio H, et al. Nephron-sparing surgery for renal tumor: a choice of treatment in an allograft kidney. Transplant Proc. 2006;38:1359-1362. [ Links ]
*12. Kunikata S, Imanishi M, Akiyama T, Kurita T. Comparative study of renal carcinomas in renal transplant, dialysis, and general (nongrafted and nonuremic) patients. Transplant Proceed. 2000; 32: 1986-1987. [ Links ]
13. Leonardou P, Semelka RC, Mastropasqua M, Kanematsu M, Woosley JT. Renal cell carcinoma in a transplanted kidney: MR imaging findings. Magn Reson Imaging. 2003; 21(6): 691-693. [ Links ]
**14. Goeman L, Joniau S, Oyen R, Van Poppel H. Percutaneous ultrasound-guided radiofrequency ablation of recurrent renal cell carcinoma in renal allograft after partial nephrectomy. J Urol. 2006; 67(1): 199.e17-199.e19. [ Links ]
15. Schostak M, Heicappell R, Sauter T, Goessl C, Krause H, Hoyer J, et al. Renal cell carcinoma in a kidney transplant: allogenic genome in the tumor justifies organ-preserving surgery. Transplant Proc. 2002; 34: 2231-2232. [ Links ]
*16. Aron M, Hegarty NJ, Remer E, O'Malley C, Goldfarb D, Kaouk JH. Percutaneous radiofrequency ablation of tumor in transplanted kidney. Urology. 2007 Apr; 69(4):778.e5-778.e7. [ Links ]
**17. Shingleton WB and Sewell PE. Percutaneous cryoablation of renal cell carcinoma in a transplanted kidney. BJU International. 2002; 90: 137-138. [ Links ]
18. Thomalla JV. Renal cell carcinoma in a renal allograft: successful treatment with 5 year follow-up. Clin Med Res. 2004; 2(3): 151-153. [ Links ]
19. Baughman SM, Sexton WJ, Glanton CW, Dalrymple NC, Bishoff JT. Computerized tomography guided radio frequency ablation of a renal cell carcinoma within a renal allograft. J Urol 2004; 172: 1262-1263. [ Links ]
20. Park KI, Inoue H, Kim CJ, Tomoyoshi T. Nephron sparing surgery for de novo renal cell carcinoma in an allograft kidney: a case report. Int J Urol. 1997; 4(6): 611-614. [ Links ]
*21. Barama A, St-Louis G, Nicolet V, Hadjeres R, Daloze P. Renal cell carcinoma in kidney allografts: a case series from single center. Am J Transplant. 2005; 5: 3015-3018. [ Links ]
22. McHayleh W, Morcos JP, Wu T, Shapiro R, Yousem S, Appleman L, Friedland DM. Renal cell carcinoma from a transplanted allograft: two case reports and a review of the literature. Clin Genitourin Cancer. 2008 Mar; 6(1):53-55. [ Links ]
*23. Ishikawa N, Tanabe K, Tokumoto T, Shimmura H, Yagisawa T, Goya N, et al. Clinical study of malignancies after renal transplantation and impact of routine screening for early detection: a singlecenter experience. Transplant Proc. 2000; 32(7): 1907-1910. [ Links ]
24. Huang S, Houghton PJ, et al. Inhibitors of mammalian target of rapamycin as novel antitumour agents. Curr Opinion in Invest Drugs. 2002; 3(2):295-304. [ Links ]
25. Tang S, Cheng IK, Leung VK, Kuok UI, Tang AW, Wing Ho Y, et al. Successful treatment of hepatitis C after kidney transplantation with combined interferon alpha-2b and ribavirin. J Hepatol. 2003; 39: 875-878. [ Links ]
26. Lledo-Garcia E, Duran-Merino R, Moralejo-Garate M, Monzo JI, Hernandez-Fernandez C. Sub-capsular nephron-sparing surgical approach for small renal graft tumor: a case report. Trans Proc. 2006; 39: 303-304. [ Links ]











 texto en
texto en