Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 supl.5 Madrid sep. 2013
CONSENSUS DOCUMENT AND CONCLUSIONS
Update on pharmacology of obesity: Benefts and risks
Farmacología de la obesidad al día: beneficios y riesgos
Lucio Cabrerizo García1, Ana Ramos-Leví1, Carmen Moreno Lopera2 and Miguel A. Rubio Herrera1
1Servicio de Endocrinología y Nutrición. Hospital Clínico San Carlos. IDISSC. Facultad de Medicina. Universidad Complutense. Madrid. Spain
2Centro de Salud Lucero. Madrid. Spain
ABSTRACT
The prevalence of obesity in Western countries has increased at a much greater pace than the development of new efficient and safe drugs, beyond mere lifestyle changes, for the treatment of overweight. Numerous different types of drugs which had been used in the past for the treatment of obesity have currently been withdrawn due to undesirable long-term side effects. The only available drug in Europe is orlistat, which serves only as an aid for the treatment of obesity. In the USA, however, a few central adrenergic-mediators, for instance, diethylpropion and phentermine, have been available for decades to treat obesity during a short-term period (less than 12 weeks). The Food and Drug Administration (FDA) has recently approved lorcaserin and the combination phentermine/topiramate for the treatment of obesity. The first one is a selective serotonin 2C receptor agonist that works by decreasing food intake with few side effects. Its outcomes on weight are modest, but may be helpful in certain selected patients. The phentermine/topiramate combination has proved to be highly effective, achieving a 10% reduction in weight in the majority of patients, although attention must be drawn to the possible development of side effects in both the short and the long-term follow-up. Further investigation regarding the mechanisms involved in weight balance will anticipate the development of new expectations for the treatment of obesity in the near future.
Key words: Obesity. Pharmacology. Pharmacotherapy.Phentermine topiramate. Lorcaserine.
RESUMEN
El incremento de la prevalencia de obesidad en los países occidentales no ha sido paralela al desarrollo de nuevos fármacos eficaces y seguros a largo plazo para el tratamiento del exceso de peso más allá de los cambios en el estilo de vida. La larga lista de fármacos que se han utilizado para el tratamiento de la obesidad han tenido que ser retirados por efectos secundarios indeseables para la salud a largo plazo. En Europa solo contamos con orlistat, como único fármaco coadyuvante para el tratamiento de la obesidad, mientras que en EEUU hace décadas disponen de unos pocos fármacos adrenérgicos de acción central (como Dietilpropion o Phentermine) para un tratamiento a corto plazo (inferior a 12 semanas). Recientemen te, la Food and Drug Administration (FDA), acaba de aprobar la lorcaserina y la combinación de Phentermine y topiramate. Lorcaserine es un agonista específico del receptor serotoninérgico 2c, con actividad anorexígena y pocos efectos secundarios. Sus efectos sobre el peso son moderados, pero pueden ser de utilidad en algunos pacientes seleccionados. La combinación de phentermine y topiramate es muy eficaz alcanzando un 10% de pérdida de peso en una mayoría de pacientes, aunque debemos estar atentos acerca de sus potenciales efectos secundarios a corto y largo plazo. La profundización en la investigación de los mecanismos implicados en la regulación del peso corporal conllevará nuevas expectativas de tratamientos para la obesidad en un futuro próximo.
Palabras clave: Fármacos obesidad. Combinación topiramato-fentermina. Lorcaserina.
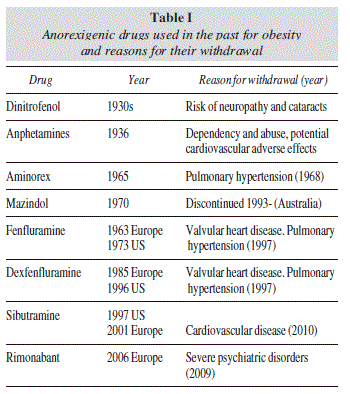
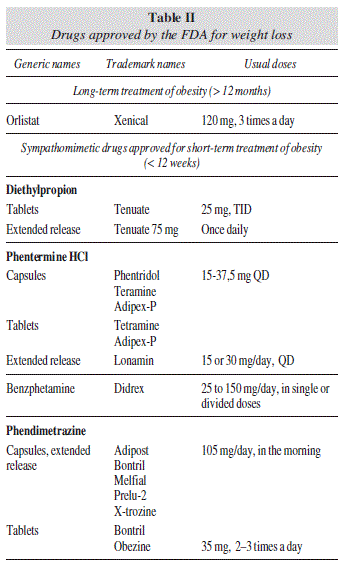
The historical progress of drug therapy for obesity has been discouraging, since no drug has achieved a long-term favorable benefit-risk ratio. Beginning with the first thyroid hormone extracts that were used in the late 19th century, continuing with amphetamines and other sympathetic-activator drugs, and the most recent sibutramine and rimonabant, both with central and peripheral action, side effects have repeatedly outweighed benefits, forcing international regulatory agencies to subsequently proceed to their withdrawal1 (table I). With the exception of orlistat, which is still available for the long term treatment of obesity (> 12 months), only a few adrenergic drugs are accessible exclusively in the USA for the short-term treatment of obesity, i.e., < 12 weeks (table II).
Undoubtedly, obesity entails a major healthcare problem which affects 24% of adult Spanish population2 and for which no real effective methods are available for its management. Conventional approaches based on lifestyle interventions reach, at the most, 5-10% weight loss at 6-12 months' follow-up, but adherence to treatments is scarce, and weight recovery is commonly observed in the long-term.3
The search for a drug with the best benefit-risk ratio should include the achievement of at least a 10-20% weight loss, which would imply an intermediate effect between the modest outcomes of lifestyle interventions (5-10%) and those of the simplest bariatric surgery technique (20-30%). For this reason, the FDA considers that a suitable drug for the treatment of obesity should comply with the following characteristics: a) a significantly different weight loss (> 5%) in comparison to placebo after one year of treatment and b) that the number of individuals reaching > 5% weight loss is at least 35% in comparison to placebo, with differences being statistically significant. In any case, drug treatment for obesity would be indicated in those with a body mass index (BMI) ≥ 30 kg/m2 or ≥ 27 kg/m2 with obesity-associated major comorbidities.
Commercially available drugs for the treatment of obesity
In Europe, the only available drug for the treatment of obesity is orlistat. The other ones which have been already approved by the FDA in the USA are still under evaluation by the European Medicines Agency (EMA).
Orlistat
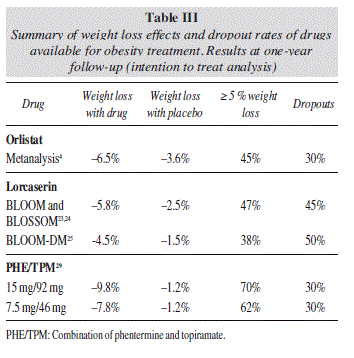
Orlistat is a gastric and pancreatic lipase inhibitor, which was first introduced in the market in 1998. Its mechanism of action is related to inhibition of fat absorption to approximately 30% of intake.4 This action entails the development of its well-known side effects, such as flatulence, increased bowel habit, voluminous stools and steatorrhea, which may occur in up to 1520% of patients; but these have not frequently been a reason for discontinuation of treatment. Orlistat 120 mg TID achieves a 3% higher weight loss than placebo and contributes to a decrease of obesity-associated metabolic comorbidities. Further subanalysis demonstrated that 26% of those receiving orlistat lost > 10% of total body weight, whilst 30% of this group lost > 5%, in comparison to 14% and 19% in the placebo group, respectively. The XENDOS (Xenical in the prevention of diabetes in obese subjects) study, a four-year double-blind placebo-controlled trial of 3305 non-diabetic obese patients, showed that orlistat reduced the risk of developing type 2 diabetes by 37.3% compared to placebo (lifestyle modification).5
Orlistat is the only currently available drug whose technical data sheet includes obesity as an indication of use. However, 32 alerts of severe hepatic failure, together with some cases of pancreatitis and renal oxalate calculi, have decreased its popularity.
Drugs that have been used to treat obesity, but that are not approved by regulatory agencies for this purpose
Bupropion
Bupropion, which inhibits the reuptake of norepinephrine and dopamine, is approved for the treatment of depression and smoking cessation. These neurotransmitters are involved as well in the regulation of food intake.6 In a study in which 327 obese subjects were randomized to placebo, bupropion 300 mg/d, or bupropion 400 mg/d, at 24 weeks, body weight was reduced by 5.0%, 7.2%, and 10.1%, respectively. The trial was extended to week 48, and weight loss in the bupropion 300-mg and 400-mg groups was 6.2% and 7.2% of the initial body weight, respectively,7 but the final dropout rate was 41%. The pharmaceutical company did not consider its commercialization for the treatment of obesity due to its central side effects, for instance, mouth dryness, insomnia, anxiety and palpitations, and due to the high discontinuation rate.
Combination of Bupropion and Naltrexone
Bupropion stimulates hypothalamic proopiomelanocortin (POMC) neurons that release alpha-melanocyte stimulating hormone ( -MSH) which, in turn, binds to melanocortin-4 receptors, and thus, favors an anorexigenic action. When α-MSH is released, POMC neurons simultaneously release β-endorphin, an endogenous agonist of the mu-opioid receptor. Binding of β-endorphin to μ-opioid receptors on POMC neurons mediates a negative feedback loop on POMC neurons leading to a decrease in the release of α-MSH. Blocking this inhibitory feedback loop with naltrexone is thought to facilitate a more potent and longer-lasting activation of POMC neurons, thereby amplifying its effects on energy balance. As a result, co-administration of bupropion and naltrexone produces a substantially greater effect on the POMC neurons, suggesting that the drugs act synergistically.
Bupropion was combined with naltrexone in its sustained release form (Contrave). Several phase III trials, grouped under the Contrave Obesity Research (COR), have been conducted in both diabetic and non-diabetic patients: COR-I, COR-II, COR-BMOD and COR-Diabetes.8-10 The naltrexone/bupropion patients lost significantly more weight (5.0% versus 1.5%, p < 0.001) at 56 weeks, with 45% of patients achieving ≥ 5% body weight loss, compared to 19% with placebo. This combination resulted in significant improvements in depressive symptoms in addition to weight loss, as well as in a satisfactory recovery of eating-control in overweight and obese women with major depression. This drug combination has generally been well tolerated in most patients; nausea was the most frequently reported adverse event, which was associated to higher naltrexone doses.
The FDA advisory panel voted 13 to 7 in favor of approval of this combination in December 2010; however, the FDA declined to approve the drug in February 2011, claiming that cardiovascular safety should be proved in a specific large-scale long-term trial, before it could be reconsidered for evaluation. This was an unexpected decision by the FDA, given that bupropion, which is the drug potentially associated with an increased cardiovascular risk, is already available and used by millions of Americans for the treatment of mild depression or smoking cessation.3
Bupropion plus zonisamide
The combination of bupropion with the antiepileptic agent zonisamide has been evaluated in phase II trials. Zonisamide's mechanism of action has not been fully characterized; however, it has demonstrated a biphasic dopamine and serotoninergic activity. A 24-week RCT of bupropion 300 mg combined with zonisamide 400 mg achieved a greater weight loss (9.2%) than either drug alone (bupropion 6.6%, zonisamide 3.6%) or placebo (0.4%). Weight loss with zonisamide and bupropion appeared to be greater than that observed with the bupropion/naltrexone combination over the same period of treatment.11,12
Topiramate
Topiramate is an anticonvulsant drug that was approved for use in certain types of epilepsy and for the treatment of migraine headache. Its mechanism of action is not fully understood, although several hypotheses are considered, such as blockage of voltage-activated sodium channels, inhibition of high-voltage-activated calcium channels, glutamate-receptor antagonism (an orexigenic agent), inhibition of carbonic anhydrase, enhancing of gamma-aminobutiric acid (GABA)-evoked currents and inhibition of kainite-evoked currents.13
It proved to reduce food intake, but was not further developed clinically because of side effects occurring at doses selected for trials. In a 6-month, placebo-controlled, dose-ranging study, 385 obese subjects were randomized to four topiramate doses: 64, 96, 192, or 384 mg/d, or placebo. The key to improve drug tolerance was that these doses were gradually increased over 12 weeks and were tapered down in a similar way at the end of the trial. Weight loss from baseline to 24 weeks was -5.0%, -4.8%, -6.3%, -6.3%, and -2.6% in the five groups, respectively.14 The most frequently reported adverse events were paresthesias (an effect due to inhibition of carbonic anhydrase), somnolence, and concentration, memory, and attention difficulties. A metanalysis of ten randomized clinical trials (3320 individuals) were recently analyzed. Patients treated with topiramate lost an average of 5.34 kg (95% confidence interval [95%CI] -6.12 to -4.56) of additional weight as compared with placebo. In the evaluation of trials using topiramate 96-200 mg day, the weight loss was higher in trials with >28 weeks of duration (-6.58 kg [95%CI -7.48 to -5.68]) than in trials with < 28 weeks (-4.11 kg [95%CI -4.92 to -3.30]).15 The authors concluded that topiramate might be a useful adjunctive therapeutic tool in the treatment of obesity as long as proper warnings about side effects are considered.
Drugs under clinical investigation
Cetilistat
Like orlistat, cetilistat is a gastrointestinal and pancreatic lipase inhibitor that reduces fat absorption. In a 12-week phase-II double-blinded RCT (n=447), cetilistat, in combination with a hypocaloric diet, produced significantly greater weight loss than placebo, with a dose-related response for 60, 120 and 240 mg TID.16 In a multicenter, randomized, double-blind study (n = 869) to determine the efficacy and safety of cetilistat (40, 80, or 120 mg TID) and orlistat (120 mg TID) in comparison to placebo, in obese patients with type 2 diabetes on metformin, for 12 weeks, similar reductions in body weight were observed in patients receiving cetilistat 80 or 120 mg TID or 120 mg TID orlistat; these reductions were significant vs. placebo (3.85 kg, 4.32 kg, and 3.78 kg, respectively; p < 0.001).17
The results are comparable to those of orlistat, with approximately 30% of the treated patients experiencing > 5% weight loss compared to 19% in the placebo group. The adverse side effects of cetilistat were similar to those reported with orlistat, although such events were less frequent, suggesting a better tolerability and therefore precluding good compliance.16,17
Tesofensine
Tesofensine is another novel pharmacological agent which inhibits the uptake of presynaptic noradrenaline, dopamine, and serotonin. Patients receiving this drug for the treatment of Alzheimer's and Parkinson's diseases reported weight loss.18 Further evidence was demonstrated in a 24-week phase IIb randomized dose-dependent tesofensine trial in 203 obese individuals, with 79% of completers.19 Weight loss was dose-dependent with 4.5% weight loss (0.25 mg), 9.2% (0.5 mg), and 10.6% (1.0 mg) and was greater than placebo (p < .0001). The drug was well tolerated, with mild symptoms appearing due to its central effects: mouth dryness, constipation, insomnia, anxiety and a significant increase in heart rate (7.4 beats/min), with no associated changes in blood pressure.19
Glucagon-Like Peptide-1 (GLP1) Analogues: Liraglutide, Exenatide
Liraglutide and exenatide are glucagon-like peptide-1 receptor analogues (GLP-1R) which were developed and approved for the treatment of type 2 diabetes. In a systematic review and metanalysis of 25 trials, GLP-1R agonist groups achieved a greater weight loss than control groups (weighted mean difference -2.9 kg, 95% confidence interval -3.6 to -2.2; 6411 participants).20 GLP-1R agonists had additional beneficial effects on systolic and diastolic blood pressure, plasma concentrations of cholesterol, and glycemic control. GLP-1R agonists were associated with nausea, diarrhea and vomiting, but not with hypoglycemia. In a head-to-head comparison, liraglutide 1.2 mg and exenatide produced similar amounts of weight loss (3.24 kg with liraglutide vs 2.87 kg with exenatide); although with both treatments 17% of patients achieved a > 5% weight loss, with liraglutide 1.8 mg/d, this rate increased to 24% of patients.21
A recent 20-week multicenter European dose-ranging RCT of liraglutide (1.2, 1.8 mg, 2.4 mg, 3.0 mg) in comparison with orlistat (120 mg) treatment in 564 non-diabetic obese patients, demonstrated a mean weight loss of 4.8 kg, 5.5 kg, 6.3 kg, and 7.2 kg, respectively, compared with 2.8 kg in the placebo-treated group and 4.1 kg in the orlistat-treated group.22 Higher doses of liraglutide (3.0 mg) demonstrated significantly greater mean weight loss than placebo or orlistat. A total of 76% achieved at least a 5% weight loss compared with 30% in the placebo group, and 44 % in orlistat group. Liraglutide reduced blood pressure at all doses, and reduced the prevalence of prediabetes (84-96% reduction) with 1.8-3.0 mg per day. Nausea and vomiting occurred more frequently in individuals on liraglutide than in those with placebo; yet adverse events were mainly transient and rarely led to discontinuation of treatment22.
Drugs approved by FDA advisory panels
The US FDA has recently approved 2 new drugs for the treatment of obesity: Lorcaserin and a combination of Phentermine plus Topiramate.
Lorcaserin (Belviq)
Lorcaserin is a selective serotonin subtype 2C receptor agonist on hypothalamic pro-opiomelancortin neurons, which leads to reduced caloric intake and increased satiety. It is similar in its mechanism of action to fenfluramine and dexfenfluramine, except for that it is specific for the 2C subtype serotonin receptor, which is not found in the heart or heart valves (these two are linked to serotonin subtypes 5A or 5B receptors). The result is thought to be a compound effect of a desirable increased satiety and an inhibition of hunger, with no heart valve damage.
The tolerability and efficacy of lorcaserin for the treatment of obesity have been evaluated in 3 large RCT, placebo-controlled, double-blind studies, which provided the basis for FDA approval in June 27, 2012. In the BLOSSOM (Behavioral Modification and Lorcaserin Second Study for Obesity Management) trial,23 4008 obese or overweight patients with obesity-related comorbid conditions were randomized to receive either lorcaserin 10 mg QD (n = 801), lorcaserin 10 mg BID (n = 1602), or placebo (n = 1601) for 52 weeks. A total of 2224 (55.5%) completed the 1-year trial. Absolute weight loss was 5.8 kg, 4.7 kg, and 2.9 kg for lorcaserin BID, lorcaserin QD, and placebo, respectively. More subjects receiving lorcaserin BID (47.2%) and lorcaserin QD (40.2%) lost at least 5% body weight at 1 year than placebo (25%), with differences being statistically significant. On the other hand, although systolic and diastolic blood pressure and heart rate decreased in all groups, differences were not statistically significant in this case.
The BLOOM (Behavioral Modification and Lorcaserin for Overweight and Obesity Management) study24 evaluated 3182 patients for up to 2 years with similar results (-5.8 kg for lorcaserin, compared with -2.2 kg for placebo). On the other hand, the BLOOM-DM (Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus) study25 evaluated the safety and efficacy of lorcaserin in 604 patients with type 2 diabetes, with glycosylated hemoglobin (HbA1c) of 7-10%, and treated with either metformin, sulfonylurea, or both. Absolute weight loss was approximately 5 kg for lorcaserin and 1.6 kg for placebo. The study found that 45 % on lorcaserin QD and 16 % on placebo achieved at least a 5% weight loss. Approximately half of the patients in the lorcaserin treatment arm achieved an HbA1c level < 7%, almost twice the rate in the placebo group. It is not clear at this time, however, whether lorcaserin has effects on glycemic control that are independent of weight loss.
On the basis of the results of these studies, lorcaserin was approved at a dose of 10 mg BID in patients with BMI ≥ 30 kg/m2, or BMI ≥ 27 kg/m2 and at least one weight-related comorbidity, such as hypertension, type 2 diabetes, or dyslipidemia, in addition to a reduced-calorie diet and an increased physical activity. This therapy should be assessed at week 12 and if there is a < 5% decrease in weight, use of the drug should be discontinued because it is unlikely that the patient will achieve and sustain an adequate weight loss with continued treatment26.
The most common adverse events with lorcaserin include headache, dizziness, nausea, constipation, fatigue, and mouth dryness. Although lorcaserin meets FDA weight loss criteria, the efficacy is modest, but the risk profile is also low. Lorcaserin treatment demonstrates an approximate average of 3 kg weight loss in a patient weighing 100 kg, or a reduction of 1.2 kg/m2 in BMI, where the basal mean BMI is 36.1 kg/m2. It is prudent to identify potential "responder patients", who will benefit from treatment, and differentiate them from other patients in which efficacy will not be likely, especially considering the fact that the estimated cost is approximately US$ 1500 per year or US$ 265 for each kilogram lost.26,27
Combination of phentermine and topiramate (Qsymia, Qsiva)
The drug combination of phentermine and topiramate (PHEN/TPM) was also recently approved by the FDA. Phentermine is a central noradrenergic drug that was commercialized in 1956 as monotherapy (15-30 mg/d) to induce a decreased appetite and favor weight loss in obese patients, but only for short-term use (< 12 weeks). Topiramate monotherapy (200-400 mg/d) was approved in 1996 for the treatment of partial seizures and in 2004 for migraine prophylaxis. The combination of PHEN/TPM allows a lower dose of both phentermine and controlled-release topiramate, and thus provides a more acceptable side-effects profile. The tolerance and safety of this drug combination are being evaluated in several phase III trials, comparing different dosages PHEM/ TPM: low-dose (3.75/23 mg), mid-dose (7.5/46 mg), and high-dose (15/92 mg).
The EQUIP trial (Controlled-Release Phentermine/ Topiramate in Severely Obese Adults: A Randomized Con trolled Trial)28 included people aged 18 to 70 years with a BMI of ≥ 35 kg/m2 who were randomized to PHEN/TPM 15 mg/92 mg (n = 512), PHEN/TPM 3.75 mg/23 mg (n = 241), or placebo (n = 514) for 52 weeks. The percentage of weight loss with high-dose was 10.9% (-12.6 kg), compared with 5.1% (-6 kg) in the low-dose and 1.6 % (-1.8 kg) for placebo (p < 0.001 for both doses compared with placebo).
The CONQUER (Effects of Low-Dose, Controlled-Release, Phentermine Plus Topiramate Combination on Weight and Associated Comorbidities in Overweight and Obese Adults) trial29 (n = 2487) compared full-dose and mid-dose PHEN/TPM with placebo for 56 weeks, including obese and overweight adults (BMI, 27-45 kg/m2) who had 2 or more weight-related comorbidities. This trial was followed by the SEQUEL study, an extension for 1 additional year.30 At the end of the CONQUER study, the percentage weight loss was 9.8% (10.2 kg) for high-dose, 7.8% (8.1 kg) with mid-dose, and 1.2% (1.8 kg) for placebo (p < 0.001 for both doses vs placebo). Approximately 70%, 62% and 21%, reached at least 5% weight loss, respectively. Weight loss was maintained during the second year of treatment in completers (SEQUEL study), resulting in 10.5% (10.9 kg) in PHEN/TPM 15/92 mg, 9.3% (9.6 kg) in PHEN/TPM 7.5/46 mg, and 1.8% (2.1 kg) in the placebo group (p < 0.001 for both dosages vs placebo). Nearly 80% of participants receiving the top-dose attained 5% weight loss compared to 75% receiving mid-dose and 30% receiving placebo (p < 0.001 for both doses compared with placebo). The combination of PHE/TPM achieves a high rate of adherence to treatment, similar to that obtained with orlistat, but with a clearly better efficacy (table III)
Following the outcomes of the results of these studies, PHEN/TPM was approved for the treatment of obesity in patients with a BMI ≥ 30 kg/m2 or a BMI ≥ 27 kg/m2 with at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus, or dyslipidemia, in addition to a reduced calorie diet and increased physical activity. Drug combination PHEN/ TPX therapy is started at 3.75 mg/23 mg, taken QD in the morning and, after 14 days it can be increased to 7.5 mg/ 46 mg. If at 12 weeks follow-up at least 3% weight loss has not been obtained, the drug may be discontinued or the dose may be increased to 11.25 mg/69 mg for another 14-day trial, followed by a final dosage increase to 15 mg/92 mg. Weight loss should be evaluated after a period of 12 weeks, and if 5% weight loss is not achieved, therapy should be withdrawn. Annual estimated cost is approximately US$ 2200 (US$ 180 per each kilogram lost).26
The most common adverse drug reactions include paraesthesias, dizziness, dysgeusia, insomnia, constipation, and mouth dryness. Potential safety concerns include depression, anxiety, cognitive-related complaints (memory and attention), cardiovascular risk with a small increase of heart rate, reduced bicarbonate levels, which could exacerbate metabolic acidosis, and teratogenicity.3
The combination of PHEN/TPM received a vote of 20 to 2 in favor at the February 2012 FDA Advisory Panel and was FDA approved on July 2012. However, on October 18th 2012, the Committee for Medicinal Products for Human Use (CHMP), from the European Medicines Agency, adopted a negative opinion and rejected marketing authorization for the medicinal product Qsiva, intended for the treatment of obesity. The CHMP remarked that main studies anticipated concerns regarding certain adverse effects related to cardiovascular risk, as well as in the psychiatric and cognitive fields; the Committee perceived that there was a high probability that, if approved, the drug would not be used strictly and exclusively for the intended patients. The applicant did propose specific measures to reduce this risk, but they were considered to be of difficult implementation in clinical practice. Therefore, the CHMP concluded that the benefits of Qsiva did not outweigh its risks and recommended that it were refused for marketing authorization.
Conclusions
Overweight and obesity are constant queries demanded in every day clinical practice, and attention to these issues should not be disregarded, since their associated comorbidities entail a significant increased morbidity and mortality. Lifestyle interventions and bariatric surgery for selected patients are the only two approaches currently available. Obesity-targeted drug therapies used in the past have deemed unreliable and the reality is that we are actually back at the starting point: the possibility of obtaining an ideal long-term effective and safe drug is still out of reach. The improvement of knowledge regarding physiopathology and mechanisms involved in food intake regulation and weight balance will surely help the development of specific therapies which will be available in the near future.
References
1. Rubio MA, Gargallo M, Millán AI, Moreno B. Drugs in the treatment of obesity: sibutramine, orlistat and rimonabant. Publ Health Nutr 2007; 10: 1200-5. [ Links ]
2. Gutiérrez-Fisac JL, Guallar-Castillón P, León-Muñoz LM, Graciani A, Banegas JR, Rodríguez-Artalejo F. Prevalence of general and abdominal obesity in the adult population of Spain, 2008-2010: the ENRICA study. Obes Rev 2012; 13: 388-92. [ Links ]
3. Wyatt HR. Update for treatment strategies of obesity. J Clin Endocrinol Metabol 2013 (Epub ahead of print February 26, 2013 as doi:10.1210/jc.2012-3115). [ Links ]
4. Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight:updated meta-analysis. BMJ 2007; 335: 1194-9. [ Links ]
5. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study. Diabetes Care 2004; 27: 155-61. [ Links ]
6. Gadde KM, Parker CB, Maner LG, Wagner HR, Logue EJ, Drezner MK, et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese Women. Obes Res 2001; 9: 544-51. [ Links ]
7. Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O'Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res 2002; 10: 633-41. [ Links ]
8. Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab 2009; 94: 4898-906. [ Links ]
9. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadaira M, Erikson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376: 595-605. [ Links ]
10. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011; 19: 110-20. [ Links ]
11. Gadde KM, Yonish GM, Foust MS, Wagner HR. Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. J Clin Psych 2007; 68: 1226-9. [ Links ]
12. Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for Obesity: Past, Current, and Future Therapies. J Obes 2011. doi:10.1155/2011/179674. [ Links ]
13. Astrup A, Toubro S. Topiramate: a new potential pharmacological treatment for obesity. Obes Res 2004; 12: 167S-73. [ Links ]
14. Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res 2003; 11: 722-33. [ Links ]
15. Kramer CK, Leitä o CB, Pinto LC, Canani LH, Azevedo MJ, Gross JL. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev 2011; 12: e338-47. [ Links ]
16. Kopelman P, Bryson A, Hickling R, Rissanen A, Rossner S, Toubro S, et al. Cetilistat (ATL-962), a novel lipase inhibitor: a 12-week randomized, placebo-controlled study of weight reduction in obese patients. Int J Obes 2007; 31: 494-9. [ Links ]
17. Kopelman P, Groot GH, Rissanen A, Rossner S, Toubro S, Palmer R, et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity 2010; 18: 108-15. [ Links ]
18. Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson's or Alzheimer's disease. Obesity 2008; 16: 1363-9. [ Links ]
19. Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 378: 1906-13. [ Links ]
20. Visboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012; 344: d7771. [ Links ]
21. Niswender K, Pi-Sunyer X, Buse J, Jensen KH, Toft AD, Russell-Jones D, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes, Obes Metabol 2013; 15: 42-54. [ Links ]
22. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009; 374: 1606-16. [ Links ]
23. Fidler MC, Sánchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011; 96: 3067-77. [ Links ]
24. Smith SR, Weissman NJ, Anderson CM, Sánchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010; 363: 245-56. [ Links ]
25. O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sánchez M, Zhang J, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity 2012; 20: 1426-36. [ Links ]
26. Chan EW, He Y, Chui CSL, Wong AYS, Lau WCY, Wong ICK. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev 2013; doi: 10.1111/obr.12015. (Epub ahead of print 2013 Jan 21). [ Links ]
27. Taylor JR, Dietrich E, Powell JG. New and emerging pharmacologic therapies for type 2 diabetes, dyslipidemia, and obesity. Clin Ther 2013; 35: A3-17. [ Links ]
28. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/ topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 2012; 20: 330-42. [ Links ]
29. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebocontrolled, phase 3 trial. Lancet 2011; 377: 1341-52. [ Links ]
30. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012; 95: 297-308. [ Links ]
![]() Correspondence:
Correspondence:
Miguel A. Rubio Herrera.
Servicio de Endocrinología y Nutrición.
Hospital Clínico San Carlos.
Martín Lagos, s/n.
28040 Madrid.
E-mail: marubioh@gmail.com