INTRODUCTION
According to the current growth standards, a small-for-gestational-age (SGA) infant is a newborn with low birth weight and/or length for gestational age and sex. The most appropriate definition of SGA has been sought for decades. In 1967, Battaglia et al. (1) set the cutoff at the 10th percentile, and two years later Usher et al. (2) set the cutoff at the z-score of -2 SD. Subsequently, major societies such as the World Health Organization (3) and the International Society of Pediatric Endocrinology (4) have maintained different opinions, setting the cutoff at the 10th percentile and a z-score of -2 SD, respectively. There is still confusion as to the correct definition of SGA (5). It is imperative to choose the cutoff point that best identifies neonates requiring specific follow-up. In 2016, Zeve et al. (6) published a systematic review that included literature reported between the years 2010 and 2015, in which a greater number of articles used the 10th percentile definition. However, they observed that newborns with more perinatal morbidity and mortality were grouped below the z-score of -2 SD or the 3rd percentile. This idea is also reflected in the work of Zhang-Rutledge et al. (7), who find a higher morbidity and mortality (resuscitation at birth, ICU admission, and perinatal death) in the group of newborns below the 5th percentile, in consideration of the group of newborns between the 5th and 10th percentiles.
In high-income countries, the incidence of SGA (using the 10th percentile definition) varies between 4.6 % and 15.3 %, with the lowest figures in Sweden, Norway, and Finland, and the highest values in Spain and Portugal (8). In low-income countries, the incidence of SGA is much higher. In the 138 countries included in the Lee et al. (9) paper, 32.4 million SGA infants were born in 2010, accounting for 27 % of all births, of which 29.7 million were term, and almost 3 million were preterm. In absolute numbers, India had the most SGA children in the world (as it registered the most births), while Pakistan had the highest percentage, with 47 % of newborns being SGA, almost one in every two births.
SGA newborns are classified into asymmetric SGA, which accounts for 70-80 % of the total, and are those with birth weight and/or length below the cutoff point but with a normal head circumference (HC), and symmetric SGA, which accounts for the remaining 20-30 % and have birth weight, length, and HC below the cutoff point. The origin of the latter occurs early in gestation, leading to a worse prognosis (10). Although the terms small for gestational age and intrauterine growth retardation (IUGR) are used interchangeably, it is worth noting there is a difference between them. The first definition of IUGR came from Warkany et al. in 1961 to describe 23 term newborns weighing less than 2000 g (11). IUGR is a dynamic term that refers to abnormal growth during gestation, while the term SGA is static and refers to weight and/or length at birth. A 2018 consensus defines IUGR as either birth weight below the 3rd percentile or three of the following criteria: birth weight below the 10th percentile, CP below the 10th percentile, length below the 10th percentile, prenatal diagnosis of IUGR, prenatal conditions associated with IUGR such as congenital infections, preeclampsia, etc. (12). A prospective longitudinal study states that the combination of fetal weight below the 3rd percentile and Doppler abnormalities in the umbilical artery could define IUGR and constitutes a poor prognostic factor (13). The most significant causes of IUGR are maternal (extreme age, race, socioeconomic level, toxic habits, arterial hypertension, preeclampsia, diabetes, assisted reproduction techniques, mother born SGA, or previous child born SGA), fetal (genetic causes, infections, congenital malformations, or metabolic diseases), and placental (placental insufficiency, chorioamnionitis) (10).
Regarding growth, we know that SGAs are born small, and some remain small into adulthood. Catch-up growth, which some SGAs (14) experience, is defined as the accelerated rate of growth that follows a period of inhibition with the aim of regaining what was lost. Different definitions of CUG have been established, such as an increase in weight and/or length above the z-score of -2 SD, the 3rd percentile, the 10th percentile, or +0.67 SD over the follow-up (15). The ideal timing of CUG is unknown, as rapid CUG has been linked to metabolic syndrome and adult cardiovascular disease, and slow CUG has been linked to growth failure and neurodevelopmental problems (16,17). Lei et al. (18) describe five growth trajectories in term SGAs from birth to seven years of age, and state that the ideal is to reach the 30th percentile in the first months of life and reach the 60th percentile at seven years of age. For their part, Shi et al. (19) also distinguish five types of trajectory in SGA growth and state that the ideal trajectory crosses two growth lines during the first months of life, from a percentile below 10 to a percentile between 25 and 50.
There are no clear guidelines to achieve this objective of slow and progressive catch-up. The different clinical practice guidelines in our setting (20) and at the international level (4) agree on the importance of identifying these newborns at risk in order to carry out adequate follow-up. There are no published data on feeding, although breastfeeding vs. formula feeding has shown better results at the metabolic profile and is considered the method of choice (21).
In any case, experiencing CUG for height does not mean acquiring the genetic height, since on average, the final height of these children remains 1 SD below their target height (22). In addition, a high percentage of SGAs have low height in adulthood (below -2 SD). This was found in a Swedish study of full-term SGAs where 7.9 % had short stature at 18 years (23). Similar data were obtained in another study where 13.6 % of full-term SGAs remained short in adulthood (24). Of all adults with short stature, in 20 % of cases the cause is being born SGA (23,25).
This work aims to: a) estimate the probability of CUG in SGA newborns at 3, 6, 12, and 48 months of life; b) observe if there are any differences in growth between different groups: symmetrical SGA newborns vs. asymmetrical SGA, and term SGA newborns vs. preterm SGA; and c) identify factors related to the absence of CUG at three months of life.
MATERIAL AND METHODS
DESIGN
This was a retrospective, longitudinal study of SGA newborns in a secondary hospital between September 12, 2011 and September 12, 2015.
INCLUSION CRITERIA
Newborns of any gestational age with birth weight and/or length at birth ≤ -2 SD were included in the study, using the growth standards of the 2010 Spanish study as reference.
DATA COLLECTION
All newborns with an SGA diagnosis born between September 2011 and September 2015 were identified. Data concerning gestation and newborns were retrieved from the hospital's electronic medical record.
MEASUREMENTS
During follow-up, anthropometric measurements were obtained by nursing staff either from the hospital or the primary care center, taking the mean of two values, expressing height in centimeters and weight in kilograms. Children under two years of age were measured in the supine decubitus position and weighed sitting or lying down. In those over two years of age, both measurements were taken standing upright: at three months, six months, and one year of age naked, and at four years of age with underwear. The tools used were calibrated. The growth standards of the 2010 Spanish study were used as reference.
DEFINITIONS
Symmetrical SGAs were considered those with head circumference, weight, and length ≤ -2 SD. Asymmetrical SGAs were those with normal head circumference with weight and/or length ≤ -2 SD. Those SGAs with altered head circumference and only weight or length were placed in the group that their growth most resembled (symmetrical or asymmetrical SGAs).
Preterm newborns were considered those born at less than 37 weeks of gestation, and term newborns were considered those born at 37 weeks or more. In preterm newborns, the z-score values were obtained by correcting the chronological age up to two years of corrected age, for which the probable date of delivery was taken and not the date of birth.
Four groups were thus created:
− Group A1: SGA by weight (asymmetric): birth weight ≤ -2 SD. Normal head circumference and length.
− Group A2: SGA by length (asymmetric): length at birth ≤ -2 SD. Normal head circumference and weight.
− Group A3: SGA by weight and length (asymmetric): weight and length at birth ≤ -2 SD. Normal head circumference.
− Group B: SGA by weight, length, and head circumference (symmetric): weight, length, and head circumference at birth ≤ -2 SD.
PRIMARY STUDY VARIABLE
Time to reach CUG from birth, defined as a change in +0.67 SD of length throughout follow-up.
SECONDARY VARIABLES
The following variables were also collected: 1) newborn: birth weight, gestational age, sex, intrauterine growth retardation (the latter defined as SGA with echo-Doppler alterations); 2) parental: maternal age, biological mother's ethnicity, biological mother and father's height, genetic or target height, parity, previous child SGA in case of parity ≥ 2, gestational diabetes, preeclampsia (arterial hypertension and proteinuria), maternal smoking; 3) fetal: multiple pregnancy; 4) placental: maternal chorioamnionitis; 5) postnatal: perinatal pathology (intraventricular hemorrhage, patent ductus arteriosus, abdominal surgery, respiratory distress requiring CPAP ≥ 24 hours), comorbidity during follow-up, feeding during first six months of life (artificial breastfeeding, exclusive breastfeeding, mixed breastfeeding).
STATISTICAL ANALYSIS
To describe the distribution of qualitative data, absolute and relative frequencies were presented. To describe the distribution of quantitative data, mean and standard deviation or median and interquartile range were presented, according to the distribution of the data.
To answer the main objective, the Kaplan-Meier survival function of time to CUG was estimated in total and for each group — A2, A3, and B — at three months, six months, 12 months, and 48 months.
The survival functions of groups A2, A3, and B were compared using the log-rank test. Differences between term SGA and preterm SGA were also analyzed in total and stratified by group (A2, A3, and B).
A univariate analysis was performed using the chi-square test or Fisher's exact test to compare qualitative variables; Student's t-test was used to compare approximately normal quantitative variables. The nonparametric Mann-Whitney U-test was used to compare quantitative variables in the absence of normality.
In response to the last objective, in which the aim was to study the possible factors associated with the absence of CUG for height at three months, the relative risks (RR) of the different factors under study were estimated. A modified Poisson (26) regression was used to fit these models.
All tests were considered bilateral, and p values < 0.05 were considered significant.
The study was approved by the Clinical Research Ethics Committee (CEIC) of the center.
RESULTS
DESCRIPTIVE RESULTS
During the study period, 384 SGA newborns were obtained from a total of 5,585 live newborns, of which 22 were excluded due to lack of access to anthropometric data, and four were excluded because anthropometries were not available before three months of life, resulting in a final sample of 358. Of these, 186 were females (52 %) and 172 were males (48 %), and 34 were preterm (9.5 %).
Of all 358, 42 presented data of IUGR with an altered echo-Doppler (11.7 %).
There were 25 symmetric SGAs (7 %), 60 weight-asymmetric SGAs (16.7 %), 200 length-asymmetric SGAs (55.9 %), and 73 weight- and length-asymmetric SGAs (20.4 %).
Of all SGAs, 15 presented comorbidity during follow-up: patent ductus arteriosus with surgical closure in two cases, atrial septal defect with surgical closure in one case, bronchopulmonary dysplasia in three cases, hemolytic disease of the newborn in one case, necrotizing enterocolitis in one case, and prolonged hospitalization (> 1 month) in seven cases. In addition, eight were diagnosed with genetic alterations and were not excluded from the study because they had auxologic behavior similar to the rest of the patients: osteogenesis imperfecta, hereditary spherocytosis, sickle cell anemia, neurofibromatosis type 1, Angelman's syndrome, nephrogenic diabetes insipidus, Léri-Weill syndrome, and microcephaly + severe pulmonary valvular stenosis syndrome.
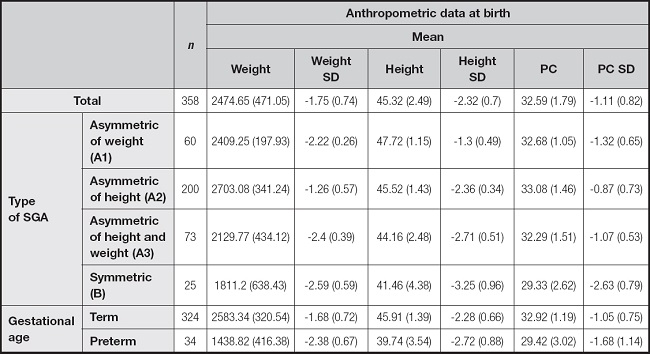
At birth, on average, preterm infants have the lowest weight and length values, while symmetric SGAs (which included term and preterm infants) have the worst mean SD in both weight and height (Table I).
Table I. Mean of anthropometric data at birth of all SGA and by groups. Weight is expressed in grams; height and PC in centimeters
In total, 13 SGAs did not undergo CUG at four years (3.6 %), and 20 SGAs remained with a height z-score at four years < -2.5 SD, of which only six received GH.
ANALYTICAL RESULTS
-
− In the group of all SGAs, we observed that most achieved CUG during the first three months of life or CE, and those who did not achieve CUG during this time rarely achieved it later.
In this group CUG was experienced at three months of life or corrected age (CA) (88 %), at six months of life or CA (93.6 %), and at 12 months of life or CA (95.3 %). At 48 months of life, 96.4 % of SGA infants achieved CUG (345/358) (Fig. 1) (Table II).
-
− When comparing the different types of SGA, we observed that symmetrical SGAs (group B) had worse length CUG results, and this was true at all ages.
In group A2 (asymmetric of length), CUG occurred at three months of life or CA (92 %), at six months of life or CA (94 %), and at 12 months of life or CA (95 %); in group A3 (asymmetric of weight and length), CUG occurred at three months of life or CA (80.8 %), at six months of life or CA (94.5 %), and at 12 months of life or CA (94.5 %); in group B (symmetric) at three months of life or CA (72 %), at six months of life or CA (84 %), and at 12 months of life or CA (92 %) (Fig. 2) (Table II).
-
− When comparing SGA by gestational age, we observed that preterm SGAs obtained worse length CUG results at all ages. This difference in success was maintained in the analysis by subgroups (A2 term vs. A2 preterm, A3 term vs. A3 preterm, and B term vs. B preterm), with the worst growth outcomes in preterm group B, i.e., symmetrical and preterm SGA.
They made length CUGs in the term SGA group at three months of age (90.4 %), at six months of age (94.1 %), and at 12 months of age (95.7 %); and in the preterm SGA group at three months of CA (64.7 %), at six months of CA (88.2 %), and at 12 months of CA (91.2 %) (Table II).
By subgroups, in subgroup A2 at 12 months of life or CA, 95.3 % of full-term SGAs (95 % CI, 91.6 %-97.7 %) and 88.9 % of preterm SGAs (95 % CI, 61.2 %-94.4 %) experienced CUG for height. In subgroup A3 at 12 months, CUG rate was 94.7 % in term SGAs (95 % CI, 86.8 %-98.6 %) and 93.8 % in preterm SGAs (95 % CI, 73.3 %-99.6 %). And finally, in subgroup B at 12 months, CUG rate was 94.1 % in term SGAs (95 % CI, 76.5 %-99.6 %), and 87.5 % in preterm SGAs (95 % CI, 57.7 %-99.3 %) (Fig. 3).
-
− Regarding the possible factors associated with the absence of CUG of length at three months, up to six variables were identified by univariate analysis.
They were prematurity with RR of 3, being symmetric SGA with RR on being weight-asymmetric of 3.4, presenting IUGR with RR of 3.9, having maternal preeclampsia with RR of 3.7, having a previous SGA child with RR of 3.1, presenting perinatal pathology with RR of 7.7, and presenting comorbidity during follow-up with RR of 4.5 (Table III)
DISCUSSION
This study is the only one published so far in our environment on the longitudinal growth of SGA newborns. In our study, the incidence of SGA newborns was 6.87 %, lower than the 15.3 % previously described in Spain (8). This is probably because we used the definition of SGA as weight and/or height below a z-score of -2 SD. The previous study used the 10th percentile as their cutoff point.
On the other hand, we also found a lower number of symmetrical SGAs, 7 % of the total number of SGAs, compared to what has been previously described in the literature, close to 20-30 % (10), which can be explained by placing 15 unclassifiable SGA neonates (with altered CP + only weight or length) in the asymmetrical SGA group.
Regarding the timing of CUG, we obtained more favorable results than those reported in the literature, with a CUG rate at six months of 93.6 % and one at four years of 96.4 %, highlighting that CUG is mostly achieved during the first months of life; if not achieved at that age, it is rarely achieved later. The study with the most similar results belongs to Huang et al. (27), where 97.3 % of full-term SGAs achieve CUG before the age of two years. In other studies, the time of CUG (being defined as overcoming -2 SD) also takes place during the first months of life, although less favorable data are shown. In the work of Hokken-Koelega et al. (28), only 87.5 % of term SGAs had achieved CUG at two years of age, while in the Swedish work of Kalsberg and Albertsson the following term SGAs had achieved CUG: 86.6 % (23) at one year of life, 92 % (29) at two years of life, and 88 % (30) at two years of life. The CUG results of our study, which are more favorable than in most published studies, may be explained because the group of preterm infants was more than 28 weeks of gestation with less comorbidity than very extremely preterm infants. Further, there was a low percentage of symmetrical SGAs, which usually show worse results. Also, we chose as CUG criterion an increase by 0.67 standard deviations along follow-up, which is easier to achieve than others.
Regarding growth in subgroups, in our study we observed that symmetric SGAs achieved less CUG and did so later than asymmetric SGAs, which has also been described in the literature. Maciejewski et al. (31) compare the percentage of CUG at nine months between symmetrical and asymmetrical term SGA neonates, with results being 70 % for the former and 85 % for the latter. The same occurs in the work of Kaur et al., which compares the height of symmetrical and asymmetrical term SGA newborns during the first year of life, with results being at birth, one month, three months, six months, nine months, and 12 months lower in the symmetrical group in both females and males, with statistically significant differences at all ages in males and only at birth and one month in females (32). This is because symmetric SGAs are newborns who have been subjected to an unfavorable environment with early intrauterine growth restriction from the first trimester of gestation.
We also obtained worse CUG results in preterm SGAs with respect to term SGAs due to the greater morbidity presented by preterm infants. This generates a situation of delayed extrauterine growth which, added to the SGA situation, makes growth in this group worse (33,34). This finding is reflected in the work of Bocca-Tjeertes et al. (35), who compare the standard deviations at four years in a group of preterm SGA and term SGA. They found that in the former group, the SDs at that age were between -1.4 and -1.7 SD, and in the latter between -0.3 and -1.0 SD. In our study, the worst CUG outcome was for symmetrical and preterm SGAs.
In the analysis of variables related to the absence of CUG at three months, the following were identified as risk factors: prematurity, symmetric SGA, IUGR, maternal preeclampsia, previous child SGA, perinatal pathology, and comorbidity during follow-up. Mc Cowan et al. (36) find that the absence of CUG at six months is related to short stature at birth and male sex. In another study, they analyzed variables associated with the absence of CUG at three years in very low birth weight preterm infants; they found that multiparity and the height z score at 12 and 24 months were risk factors, the latter being the best predictor (37). For their part, Leger et al. (24) state that low height at birth and the height of both parents are predictors of low final height. Another study that finds maternal height important is that of Xie et al. (38), which also identifies smoking mothers with low weight gain during pregnancy as a risk factor for the absence of CUG at five years of age.
We found certain limitations. Since ours was a retrospective study, we could not choose the variables to be collected, so there is a lack of information when interpreting growth failure — for example, paternal height, a variable that should be taken into account in subsequent studies, as it has an important influence on the final height of the child. In addition, the measurements were not taken by the same person, which could lead to some interindividual variability. Certain rules were respected, such as using the same calibrated tools, with the patient naked and at the same ages. Another limitation is that the sample size was reduced by choosing “weight and/or height below -2 SD” as SGA criterion instead of other criteria that are more frequently met by newborns, for example, “weight and/or height below p10.” Choosing the cutoff at -2 SD reduced our sample but had the strength of picking up the most at-risk SGAs, which have a different behavior.
CONCLUSIONS
The majority of SGA infants show adequate CUG during the first months of life, leaving a small percentage of these, especially premature and symmetrical SGAs, with insufficient growth and low height in adulthood.
The study highlights the importance of knowing the risk factors associated with the absence of CUG in order to identify the most at-risk newborns and establish appropriate follow-up and management. Furthermore, there is a need to homogenize the criteria that define SGA and CUG, and to develop prospective longitudinal studies that will help us better understand growth in these children.




















