Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Anales de Psicología
versión On-line ISSN 1695-2294versión impresa ISSN 0212-9728
Anal. Psicol. vol.31 no.2 Murcia may. 2015
https://dx.doi.org/10.6018/analesps.31.2.166361
The longitudinal measurement of change: Intraindividual variability in behavior and interindividual differences observed in childhood
Diseños de observación longitudinales: cambio intra-individual y diferencias inter-individuales observados durante la infancia
Elena Escolano-Pérez1 y Ángel Blanco-Villaseñor2
1 Universidad de Zaragoza (Spain).
2 Universidad de Barcelona (Sapin).
This study forms part of research funded by a grant [DEP2012-32124] from the State Secretariat of Research, Development and Innovation of the Spanish Ministry of Economy and Competitiveness for the period 2012-2015.
ABSTRACT
The study of change in repeated measures studies or longitudinal studies (cross-sectional and/or cross-sequential) is of considerable interest in the field of developmental psychology. Qualitative and quantitative measures of interindividual and intraindividual variability can be used to capture changes in cognitive development.
In the present study, through an empirical analysis of infant cognitive development, we investigate whether or not longitudinal (cross-sectional/cross-sequential) research designs can be used interchangeably with univariate or multivariate data analysis techniques. Methodologically, longitudinal data can be processed by univariate or multivariate analysis. However, the results and their interpretation may be different, even when the necessary statistical requirements are performed. Current statistical programs incorporate techniques to test for the presence of significant differences in data, regardless of whether these are evaluated by univariate or multivariate analysis.
The results of this study, conducted in infants studied at three time points (18, 21 and 24 months), show that both intraindividual and interindividual variability can be detected by repeated measures analyses.
Key words: Development; childhood; variability; longitudinal.
RESUMEN
El estudio del cambio en estudios de medidas repetidas o estudios longitudinales (cross-sectional y/o cross-sequential) constituye un area de notable interés en el ámbito de la Psicología del Desarrollo. Las medidas (cualitativas/cuantitativas) que tomamos de participantes, bien intraindividualmente bien interindividualmente, permiten capturar cambios del desarrollo cognitivo.
Mediante un estudio empírico de desarrollo cognitivo infantil, comprobaremos si los diseños longitudinales (cross-sectional/cross-sequential) pueden utilizarse o no indistintamente con técnicas de análisis de datos uni- o multivariables. Metodológicamente es posible tratar datos longitudinales en alguna de las soluciones aportadas, uni o multivariable. Sin embargo, y aun cumpliendo los requisitos estadísticos necesaRíos, los resultados y la interpretación de los mismos pueden ser diferentes.
Entendemos que hay soluciones en los programas estadísticos actuales que permiten la utilización de técnicas que aseguren si realmente hay diferencias significativas o no en los datos, independientemente de si son tratados con estructuras uni- o multivariables.
Los resultados de bebes estudiados en tres momentos temporales (18, 21 y 24 meses) mediante medidas repetidas muestran que la interpretación afecta de igual manera a datos de la variabilidad intra/interindividual.
Palabras clave: Desarrollo; infancia; variabilidad; longitudinal.
Introduction
The main aim of human development sciences is to determine how, when, and why individuals change, in the broadest sense of the word (i.e. neural development, behavior, emotional experiences, etc.) (Baltes & Nesselroade, 1979). This is no easy task, however, considering the complexity of human development and all this implies. As stated by Siegler and Crowley (1991, p. 606), "the essence of development is change".
Human development is a complex process of constant, multifaceted, multidimensional, and multidirectional change with multiple causes and consequences. "Who we are and what we think is a product not only of our genes, but also of our social, cultural, and physical environments, of their interactions with one another, and of their interactions with our genes" (Diamond, 2007, p. 152). Considering thus the dynamic interplay of multiple, changing elements involved in human development (Diamond, 2009; Karmiloff-Smith, 2009; Mareschal, 2011), it is only logical that this process is also characterized by multiple and diverse modes of interaction and results. In other words, human variability is an inherent part of development (Torbeyns, Arnaud, Lemaire & Verschaffel, 2004; van Dijk & van Geert, 2011). Furthermore, as stated by Puche and Martí (2011, p. 134), "variability is the gateway to development studies, the basis for explaining development processes ". This has not always been considered to be the case, however.
Developmental psychology studies have traditionally focused on searching for regularities and gradual, normative aspects of change that help to describe general development patterns. Differential psychology, however, emerged as a new discipline that turned the spotlight on human behavior (Nesselroade, 2002). For many years, universality and variability had been viewed as two opposing forces. This dichotomous view, however, is erroneous.
Baltes, Reese and Nesselroade (1977) defined development research as the search for interindividual differences and similarities in patterns of intraindividual change. Developmental psychology concerns not only normative changes that occur over a life time, but also differences and variations in individual lives-development variability-and biological and cultural conditions that give rise to unique courses of development.
It is therefore evident that human diversity and variability are both now viewed as an inherent part of development. To study change or development thus, it is necessary to study human variability. As remarked by Posner, Rothbart and Sheese (2007, p. 24), "we need to understand both common processes and individual differences" in the study of development as they all constitute the object of study. General or universal patterns and differences should therefore be seen as complementary rather than opposing forces.
These conceptual advances in developmental psychology have been accompanied by methodological advances, including the introduction of new concepts, models and procedures that seek to capture change and contribute to our understanding of human development in all its dimensions and diversity throughout the life cycle. This interdependence between the view of human development and methodology is becoming increasingly clear (Puche & Martí, 2011).
While classic methods sought to minimize variability (by focusing on means) and depict development as a regular, linear process, new methods not only recognize the inherent variability of development but have also made it the object of analysis through the modeling of true development curves that are generally complex and non-linear (Cheshire, Muldoon, Francis, Lewis & Ball, 2007; Grimm, Ram & Hamagami, 2011; Lavelli, Pantoja, Hsu, Messinger & Fogel, 2005).
Cognitive and neuropsychological research has traditionally focused on comparing mean results, eclipsing research into intraindividual variability (Nesselroade & Molenaar, 2010; Nesselroade & Ram, 2004). Such an approach represents a considerable simplification of behavioral patterns that can lead to erroneous inferences (Nesselroade, 2002). Recognition of these shortcomings, however, has given rise to models that explain performance based on multiple distribution parameters rather than on exclusive measures of central tendency. The incorporation of variance parameters yields predictive information about cognitive functioning regardless of mean performance (West, Murphy, Armilio, Craik & Stuss, 2002), with specific analyses of discrimination between groups to facilitate the detection of variability across multiple dimensions.
It is essential to account for the multiple dimensions that comprise development, as development markers can follow different patterns (Granott & Parziale, 2002; van Dijk & van Geert, 2011). The analysis of multiple indicators yields more information on the different elements that comprise the actual process of change, which is why considering these elements as a whole obscures the very nature of development. The combined use of different approaches adapted to both the sample and the indicator(s) being analyzed helps to achieve a better and more adequate understanding of cognitive changes (Cheshire et al., 2007).
In short, following a period dominated by an emphasis on universal aspects of human development, the study of intra-and interindividual differences is clearly gaining ground in cognitive developmental psychology. This is particularly patent in studies that deal with infant cognitive development, as "the development of cognitive strategies during childhood is not a linear process in which these emerge progressively, without fluctuations or set-backs, but rather, as shown by numerous empirical studies (...), the process by which cognitive strategies emerge and become established is highly irregular" (García-Mila, Gilabert & Rojo, 2011; p. 169). These changes reflect the attempts made by the child to coordinate skills, motivations, and the demands required by different tasks. Variability rather than uniformity is thus a characteristic of infant cognitive development (Puche & Martí, 2011; Siegler, 2007) and it is this variability that is gradually being given a central role in explaining the emergence of new capacities (van Dijk y van Geert, 2011). The study of childhood thus is crucial for understanding development (Lyra & Valsiner, 2011).
Such studies, however, require not only a solid theoretical framework for interpreting the results but also a rigorous methodological approach consisting of the collection of longitudinal data characterized by, at least, three aspects: 1) The same participants or units should be observed at repeated moments over time; 2) The same measurements should be used throughout the study; and 3) It must be known when each measurement was taken (Baltes & Nesselroade, 1979; Ferrer & Grimm, 2012; McArdle & Nesselroade, 2003).
The present study integrates theoretical, technical, and methodological aims. Our overall aim was to test for the presence of significant differences in infantile cognitive development at three time points analyzed from the intra-and interindividual perspectives using three statistical methods that have the same goal yet are different.
Missing data is a common problem in longitudinal studies of young participants (infants) and therefore groups of children analyzed at different ages (time points) may not be balanced and analyses could be affected by a considerable loss of data. Our aim was to adapt our statistical hypotheses to possible analyses of data sets with fewer missing data, as it is difficult to obtain large samples in childhood studies.
Decisions on how to address problems associated with missing data and small sample sizes are becoming increasingly important, as longitudinal studies are gaining prominence in psychological research around the world (Collins, 2006; Kuljanin, Braun & Deshon, 2011). Howitt and Cramer (2011) reported that 41% of studies in the PsycINFO database had been conducted with experimental methods, and 40% with longitudinal and qualitative methods (33% and 7% respectively). The remaining 19% used other methodologies. These figures highlight the importance of our theoretical-technical-methodological study in the area of longitudinal research in child development.
Method
Participants
The study sample was formed by 48 infants considered to have normal development: they had been born to term (37-40 weeks of gestation) and had no known diseases or associated risk factors. They were observed in a longitudinal study with repeated measures taken at three time points: 18, 21 and 24 months of age. All the participants were treated in accordance with international standards and ethical principles for scientific research.
Instruments
The material used for the stimulus exercise consisted of two sets of four cups and four balls. The balls and cups were matched by size, i.e. each ball fit in one cup. There were three tasks. Tasks 1 and 3 involved colored balls and colored cups, while 2 involved white cups and balls. Color matched size in Task 1 but not in Task 3. Task 1 was therefore considered easier than Task 3, as color is perceived and processed more rapidly than size. Task 3 was considered the most difficult task, as there was no relationship between size and color and the task could not be resolved by focusing on color alone. All the cups and balls used in Task 2 were white and therefore the exercise was more difficult than Task 1 but easier than Task 3.
The following instruments were used for image recording, digitalization, and compression: a digital video camera, a video capture/tuner card, and the software programs Adobe Premier Pro 1.5 and Mainconcept MPEG Encoder.
The data were encoded using the ELEDA (Early Logical and Executive Development Assessment; Escolano-Pérez & Sastre-Riba, 2010) observation instrument for observing executive function and logical operations in infants through the recording of the frequency of these functions and operations and the time (in seconds) they started and finished. This information was recorded using the software program Match Studio Vision v. 3.0 (Perea, Alday & Castellano, 2006).
The statistical programme SAS 9.1.3 GML Procedure and Mixed Procedure (SAS Institute Inc., 2004; Schlotzhauer & Littell, 1997) was used for the univariate and multivariate longitudinal analysis.
Procedure
The frequency and duration of the executive functions and logical operations executed spontaneously by each participant while trying to resolve each of the three tasks were recorded. The tasks were presented to the infants in increasing order of difficulty (Task 1, 2 and 3).
In each case, recording was stopped when the task was successfully completed (i.e. when the child correctly matched each ball to its corresponding cup), when the child stopped the activity, or when the activity became repetitive.
Results
Statistical models of individual development have traditionally been designed to evaluate and analyze participant data in studies in which the same experimental unit is measured at two or more points in time (longitudinal studies with a repeated measures design).
Repeated measures analyses have typically been conducted using SAS and SPPS software. The general linear model procedure in SAS (PROC GLM), however, is only valid for traditional univariate and multivariate analyses. A more recent mixed procedure developed for SAS (PROC MIXED) uses a more general covariance structure approach that is more suited to individual development models (Castellano, Blanco-Villaseñor & Álvarez, 2011; Vallejo, Fernández, Livacic-Rojas & Tuero-Herrero, 2011).
Because repeated measures analysis involves multiple measurements taken at different points in time in the same participant (development curve), the choice of using PROC GLM or PROC MIXED will depend on the advantages and disadvantages offered by each technique for the study in question.
One important consideration is that repeated observations in the same individual are generally correlated and exhibit heterogeneous variability. When this is not the case, traditional GLM procedures based on least squares analysis will be sufficient, as it is assumed that the observations are not correlated and have constant variance (homoscedasticity). If correlation and non-constant variability are observed, PROC MIXED should be used, particularly as it can be used to make inferences and generalizations about fixed effects.
Both procedures (PROC and MIXED) have analytical techniques for repeated measures that account for within-subject covariance. However, GLM PROC requires the within-subject data to be balanced, i.e. it does not permit missing data. In such cases, it is necessary to identify all participants with complete data and to define a between-subject and within-subject fixed effects model. Between-subject effects remain constant while within-subject effects vary from one individual to the next.
These requirements of PROC GLM limit many other types of analysis due to the dichotomy between between-subject and within-subject effects. The alternative to a repeated measures strategy is PROC MIXED, which enables the analysis of participants with missing data. It can do this because it uses restricted maximum likelihood estimation (REML) instead of the method of least squares -which requires complete data-employed by PROC GLM.
Tables 1 and 2 show the data from our study analyzed from two different perspectives: univariate analysis and mul-tivariate analysis. The same data were analyzed using SAS in both cases. In the first case, we used the repeated measures analysis in PROC GLM and in the second case, we performed a multivariate analysis of variance (MANOVA) in PROC GLM with transformation to multivariate data, where each of the three points analyzed was defined as a dependent variable.
In both situations, we analyzed age and the effects of the interaction age × participant.
In the univariate analysis, age did not exert a significant effect on the repeated measures taken at the three time points (.8489) (Table 1), unlike the interaction age × participant (p < .0001). This suggests that it is not age that explains the cognitive changes observed, but rather a set of participants within a given age group.
The multivariate analysis (Table 2) showed partial correlation coefficients, with weak yet significant relationships, between the three time points. We applied Mauchly's sphericity test (1940) to check that the data had a multivariate structure and that MANOVA was possible. We saw that the transformation to multivariate data and the orthogonality of the data (for age groups with a different number of participants and/or missing variables) offered significant values (p < .0001) and could therefore be processed and interpreted as multivariate data.
Finally we used MANOVA to investigate the effect of age and the interaction age × participant at the three time points studied. In the case of age only, the results were not significant for any of the four statistical analyses employed, but they were significant for the interaction between age and participant. In both cases, we ensured that the program accounted for missing data, that the data for the comparison of groups at the three time points was balanced, and that the variables were transformed into a valid multivariate structure.
Evidently, if we had only performed the first analysis (PROC GLM), even though the interpretation is similar, considering that the program automatically eliminates information for all participants with missing data and compares the number of participants in each age group at each of the three time points, we would never have known if the differences detected were not significant because of the size of the sample or the number of missing variables (and hence participants) that the program ignored.
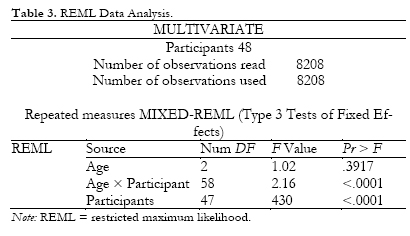
Table 3 shows the multivariate results in a fixed effects model for age, age × participants, and participants. In terms of interpretation, the information in Table 3 (while very different) is similar to that in Tables 1 and 2. Age was not significant in any of the three tables (.8489 for the univariate analysis, .1283 for the multivariate analysis, and .3517 for the multivariate-REML analysis).
The multivariate solution yielded more accurate results with the data available, regardless of the structures required by the univariate analysis.
Discussion
The results obtained by PROC GLM did not show a significant effect of age at any of the three time points analyzed in this repeated measures study. The same procedure, however, yielded significant results for the effects of the age × participant interaction, suggesting that certain participants within a given age group rather than age itself were responsible for the cognitive changes observed. These results are consistent with the current understanding of development. Age alone can no longer be considered to explain change (Schmidt & Teti, 2005). Change is the result of multiple variables that are constantly changing and interacting with each other, and it can therefore be assumed that multiple processes are at work in any individual at any time. Furthermore, this change and interaction varies from one person to the next and therefore the relationship behavior-age-capacity can no longer be considered valid (Puche & Martí, 2011). Development is not a linear process and can therefore no longer be simplified in terms of fixed, cumulative pathways.
This dynamic process makes it all the more difficult to understand how and when development occurs. However, this cannot be considered a hurdle or excuse if the goal is to build a solid scientific foundation for helping to better understand, explain, and predict human behavior, be it typical or atypical (Amso, 2011; Blanco-Villaseñor, Sastre Riba & Escolano-Pérez, 2010; Casey et al., 2009; Coch, Dawson & Fischer, 2007; Empson, 2006; Escolano-Pérez & Sastre, 2006; Horridge, 2011; Juffer, van Ijzendoorn & Palacios, 2011; Karmiloff-Smith, 2007, 2009; Tau & Peterson, 2010; Walker et al., 2007; Whelan & Mathews, 2011).
Behavioral measures have played an important role in improving our understanding of cognitive changes over time, and specifically for learning what develops and when (Amso & Casey, 2006). However, collecting and analyzing behavioral data in isolation may lead to misleading conclusions, as these data do not capture all changes in development (Rueda, 2010). Behavioral measures only reflect the 'last directly observable response", but we now know that cognitive and brain development occur in interaction (Casey, Tottenham, Liston & Durston, 2005; Diamond, 2011; Durston & Casey, 2006; Escolano-Pérez, 2013; Munakata & Johnson, 2006; Tau & Peterson, 2010).
Neuroimaging and other recent advances have come to play an important role in evaluating the biological mechanisms underlying cognitive changes. Numerous studies have shown that certain cognitive milestones achieved during the first two years of life are related to both anatomic and functional changes in the brain, and the frontal cortex in particular. These include changes in electrical activity patterns that increase the base activity; increased dopamine levels; reduced neuronal density; and increased frontal white matter (Banich & Compton, 2011; Deoni et al., 2011; Johnson, 2010)
These technological advances have given birth to a new scientific discipline, developmental cognitive neuroscience, which is a cross between developmental psychology and neuroscience that seeks to combine behavioral, electrophysiological, and neuroscientific measures to aid the understanding of cognitive processes (Blakemore, Dahl, Frith & Pine, 2011; Diamond & Amso, 2008; Pennington, Snyder & Roberts, 2007; Rodrigo, 2010).
These new techniques have made a particularly important contribution to our understanding of cognitive development as they allow complex brain-mind relationships to be broken down into multiple levels of analysis. Despite their many advantages, however, these techniques also have limitations and risks (Gómez, 2010; Oliva, 2010).
One important limitation is that they provide an indirect measurement of brain function, as, while they measure changes in degrees of activity and volume of structures, they cannot characterize the mechanisms underlying these changes, i.e. they cannot tell us whether the changes are due to dendritic branching, apoptosis, apoptogenesis, myelination, or other processes (Amso & Casey, 2006; Deoni et al., 2011; Maestú, Ríos & Cabestrero, 2008)
There is also a risk that an excessive focus on these techniques will lead to a reductionist view of development. Breaking down the object of study into multiple levels of analysis helps us to better understand the links between the brain and the mind, but this will be of little use if unaccompanied by an interdisciplinary exercise to reconstruct the chain using the knowledge and explanations found at each level of analysis (Crone & Ridderinkhof, 2011; Martí, 2010). It is unlikely that the detailed analyses offered by advanced neuroimaging technology will improve our understanding of how development starts if we lack a guiding theoretical framework. As stated by Kadosh (2011), advances from neuroimaging studies will depend on the theoretical frameworks built to guide our research (Kadosh, 2011). If, blinded by technology, we pursue an oversimplified approach, we run the risk of psychological phenomena being viewed simply in terms of neural activity.
These new technologies and methods are necessary, but their use must provide results that help to better understand the processes that link behavioral observations to developing brain circuitry (Casey, Soliman, Bath y Glatt, 2010). Furthermore, it is important to extend these new methodological approaches that have emerged in developmental psychology into new fields.
One of the limitations of the present study was not having a data set with sufficient missing data to be able to determine the effects in a multivariate analysis such as the one we propose. In our opinion, future studies should ensure that this information is available in order to be able to perform multiple imputation of missing data to examine the potential benefits of such an approach, even with information on participants who do not theoretically exist, i.e. information estimated using standard MIXED procedures in SAS.
In short, studies of human behavior need to take an interdisciplinary focus consisting of multiple levels of analysis (Forstmann, Wagenmakers, Eichele, Brown & Serences, 2011; Oliva, 2010; Rueda, 2010), because, as stated by Gottlieb (2011), it is the interaction between elements rather than the elements themselves that gives rise to development. The merging of efforts across disciplines, including not only developmental psychology and neuroscience but also developmental psychopathology, pediatrics, neuropediatrics, neurobiology, genetics, etc. (Johnson, 2011) will provide a fuller and more comprehensive explanation of processes related to human development, which, in turn, will open up promising opportunities for new forms of intervention.
References
1. Amso, D. (2011). The developmental process: Evidence from an integrating science. In J. Hakansson (Ed.), Developmental Psychology (pp. 181-184). Hauppauge, NY: Nova Science Publishers. [ Links ]
2. Amso, D., & Casey, B. J. (2006). Beyond what develops when neuroimaging may inform how cognition changes with development. Current Directions in Psychological Science, 15(1), 24-29. [ Links ]
3. Baltes, P. B., & Nesselroade, J. R. (1979). History and rationale of longitudinal research. In J. R. Nesselroade and P. B. Baltes (Eds.), Longitudinal research in the study of behavior and development (pp. 1-39). New York: Academic Press. [ Links ]
4. Baltes, P. B., Reese, M. W., & Nesselroade, J. R. (1977). Life-span developmental psychology. Monterey, CA: Brooks/Cole. [ Links ]
5. Banich, M. T., & Compton, R. J. (2011). Cognitive Neuroscience. Belmont, CA: Wadsworth Publishing. [ Links ]
6. Blakemore, S. J., Dahl, R. D., Frith, U., & Pine, D. S. (2011). Editorial. Developmental Cognitive Neuroscience, 1(1), 3-6. [ Links ]
7. Blanco-Villaseñor, A., Sastre Riba, S., & Escolano-Pérez, E. (2010). Desarrollo ejecutivo temprano y Teoría de la Generalizabilidad: bebes típicos y prematuros. Psicothema, 22(2), 221-226. [ Links ]
8. Casey, B. J., Glatt, C. E., Tottenham, N., Soliman, F., Bath, K., Amso, D., ... & Lee, F. S. (2009). Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience, 164, 108-120. [ Links ]
9. Casey, B. J., Soliman, F., Bath, K. G., & Glatt, C. E. (2010). Imaging genetics and development: Challenges and promises. Human Brain Mapping, 31, 838-851. [ Links ]
10. Casey, B. J., Tottenham, N. T., Liston, C., & Durston, S. (2005). Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences, 9(3), 104-110. [ Links ]
11. Castellano, J., Blanco-Villaseñor, A., & Álvarez, D. (2011). Contextual variables and time-motion analysis in soccer. International Journal of Sports Medicine, 32, 1-7. [ Links ]
12. Cheshire, A., Muldoon, K. P., Francis, B., Lewis, C. N., & Ball, L. J. (2007). Modelling change: new opportunities in the analysis of microgenetic data. Infant and Child Development, 16(1), 119-134. [ Links ]
13. Coch, D., Dawson, G., & Fischer, K. W. (Eds.) (2007). Human behavior, learning, and the developing brain: Atypical development. New York: Guilford Press. [ Links ]
14. Collins, L. M. (2006). Analysis of longitudinal data: The integration of theoretical model, temporal design, and statistical model. Annual Review of Psychology, 57, 505-528. [ Links ]
15. Crone, E. A., & Ridderinkhof, R. (2011). The developing brain: From theory to neuroimaging and back. Developmental Cognitive Neuroscience, 1, 101-109. [ Links ]
16. Deoni, S., Mercure, E., Blasi, A., Gasston, D., Thomson, A., Johnson, M. H., Williams, S., & Murphy, D. (2011). Mapping infant brain myelination with magnetic resonance imaging. Journal of Neuroscience, 31(2), 784-791. [ Links ]
17. Diamond, A. (2007). Interrelated and interdependent. Developmental Science, 10(1), 152-158. [ Links ]
18. Diamond, A. (2009). The interplay of biology and the environment broadly defined. Developmental Psychology, 45(1), 1-8. [ Links ]
19. Diamond, A. (2011). Biological and social influences on cognitive control processes dependent on prefrontal cortex. Progress in Brain Research, 189, 319-339. [ Links ]
20. Diamond, A., & Amso, D. (2008). Contributions of Neuroscience to our understanding of cognitive development. Current Directions in Psychological Science, 17(2), 136-141. [ Links ]
21. Durston, S., & Casey, B. J. (2006). What have we learned about cognitive development from neuroimaging? Neuropsychologia, 44(11), 2149-2157. [ Links ]
22. Empson, J. M. (2006). Factores de riesgo en el desarrollo del niño. En J. M. Empson y D. Nabuzoka (Dirs.), El desarrollo atípico infantil. Problemas emocionales y conductuales. Maltrato infantil. Problemas de caprendizaje (pp. 33-69). Barcelona: Ediciones CEAC. [ Links ]
23. Escolano-Pérez, E. (2013). El cerebro materno y sus implicaciones en el desarrollo humano. Revista de Neurología, 56, 101-108. [ Links ]
24. Escolano-Pérez, E., & Sastre, S. (2006). Actividad lógica de los bebes: un estudio diferencial. Psicothema, 18(3), 537-543. [ Links ]
25. Escolano-Pérez, E., & Sastre-Riba, S. (2010). Early infant cognitive assessment: validity of an instrument. Behavior Research Methods, 42(4), 759-767. [ Links ]
26. Ferrer, E., & Grimm, K. J. (2012). Issues in collecting longitudinal data. In H. Cooper, P. M. Camic, D. L. Long, A. T. Panter, D. Rindskopf and K. J. Sher (Eds.), APA Handbook of research methods in Psychology, Vol 2: Research designs: Quantitative, qualitative, neuropsychological, and biological (pp. 275-290). Washington, DC: American Psychological Association. [ Links ]
27. Forstmann, B. U., Wagenmakers, E. J., Eichele, T., Brown, S., & Serences, J. T. (2011). Reciprocal relations between cognitive neuroscience and formal cognitive models: opposites attract? Trends in Cognitive Sciences, 15(6), 272-279. [ Links ]
28. García-Mila, M., Gilabert, S., & Rojo, N. (2011). El cambio estratégico en la adquisición del conocimiento: la metodología microgenética. Infancia y Aprendizaje, 34(2), 169-180. [ Links ]
29. Gómez, J. C. (2010). Shadows of the living dead-potential dangers of unsafe encounters between developmental psychology and neuroscience. Infancia y Aprendizaje, 33(1), 19-24. [ Links ]
30. Gottlieb, G. (2011). Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. In K. E. Hood, C. Tucker Halpern, G. Greenberg & R. M. Lerner (Eds.), Handbook of Developmental Science, Behavior, and Genetics (pp. 13-37). Oxford: Wiley-Blackwell. [ Links ]
31. Granott, N., & Parziale, J. (2002). Microdevelopment: A process-oriented perspective for studying development and learning. In N. Granott & J. Parziale (Eds.), Microdevelopment: Transition processes in development and learning (pp. 1-28). Cambridge: Cambridge University Press. [ Links ]
32. Grimm, K. J., Ram, N., & Hamagami, F. (2011). Nonlinear growth curves in developmental research. Child Development, 82(5), 1357-1371. [ Links ]
33. Horridge, K. A. (2011). Assessment and investigation of the child with disordered development. Archives of Disease in Childhood. Education and Practice, 96(1), 9-20. [ Links ]
34. Howitt, D., & Cramer, D. (2011). Introduction to research methods in Psychology (3rd ed). Harlow: Pearson Education Limited. [ Links ]
35. Johnson, M. H. (2011). Developmental neuroscience, psychophysiology, and genetics. In M. H. Bornstein and M. E. Lamb (Eds.), Developmental Science: An advancedtextbook (6th ed, pp. 201-239). New York: Psychology Press. [ Links ]
36. Johnson, S. P. (2010). Neoconstructivism. The new science of cognitive development. New York: Oxford University Press. [ Links ]
37. Juffer, F., van Ijzendoorn, M. H., & Palacios, J. (2011). Recuperacion de niños y niñas tras su adopción. Infancia y Aprendizaje, 34(1), 3-18. [ Links ]
38. Kadosh, K. C. (2011). What can emerging cortical face networks tell us about mature brain organisation? Developmental Cognitive Neuroscience, 1 (3), 246-255. [ Links ]
39. Karmiloff-Smith, A. (2007). Atypical epigenesis. Developmental Science, 10(1), 84-88. [ Links ]
40. Karmiloff-Smith, A. (2009). Nativism versus Neuroconstructivism: Rethinking the study of developmental disorders. Developmental Psychology, 45(1), 56-63. [ Links ]
41. Kuljanin, G., Braun, M. T., & Deshon, R. P. (2011). A cautionary note on modeling growth trends in longitudinal data. Psychological Methods, 16(3), 249-264. [ Links ]
42. Lavelli, M., Pantoja, A. P. F., Hsu, H., Messinger, D., & Fogel, A. (2005). Using microgenetic designs to study change processes. In D. M. Teti (Ed.), Handbook of research methods in Developmental Science (pp. 40-65). Malden, MA: Blackwell Publishing. [ Links ]
43. Lyra, M. C. D. P., & Valsiner, J. (2011). Historicity in development: Abbreviation in mother-infant communication. Infancia y Aprendizaje, 34(2), 195-203. [ Links ]
44. Maestú, F., Ríos, M., & Cabestrero, R. (Eds.). (2008). Neuroimagen. Técnicas y procesos cognitivos. Barcelona: Masson. [ Links ]
45. Mareschal, D. (2011). From NEOconstructivism to NEUROconstructivism. Child Development Perspectives, 5, 169-170. [ Links ]
46. Martí, E. (2010). Reductionism's long shadow: commentary on "Where developmental psychology and neuroscience meet a threatening or a felicitous encounter?" by María-José Rodrigo. Infancia y Aprendizaje, 33(1), 25-28. [ Links ]
47. Mauchly, J. W. (1940). Significance test for sphericity of a normal n-variate distribution. The Annals of Mathematical Statistics, 11, 204-209. [ Links ]
48. McArdle, J. J., & Nesselroade, J. R. (2003). Growth curve analysis in contemporary psychological research. In J. Schinka and W. Velicer (Eds.), Comprehensive handbook of psychology: Research methods in psychology (Vol. 2, p. 447-480). New York: Wiley. [ Links ]
49. Munakata, Y., & Johnson, M. H. (Eds.) (2006). Attention and performance XXI: Processes of change in brain and cognitive development. Oxford: Oxford University Press. [ Links ]
50. Nesselroade, J. R. (2002). Elaborating the differential in Differential Psychology. Multivariate Behavioural Research, 37(4), 543-561. [ Links ]
51. Nesselroade, J. R., & Molenaar, P. C. M. (2010). Emphasizing intraindividual variability in the study of development over the lifespan. In W. F. Overton (Ed.), Cognition, Biology, and Methods across the Lifespan (Vol. 1, pp. 30-54). Hoboken, NJ: Wiley. [ Links ]
52. Nesselroade, J. R., & Ram, N. (2004). Studying intraindividual variability: What we have learned that will help us understand lives in context. Research in Human Development, 1 , 9-29. [ Links ]
53. Oliva, A. (2010). The powerful pull of brain images. Infancia y Aprendizaje, 33(1), 29-34. [ Links ]
54. Pennington, B. F., Snyder, K. A., & Roberts, J. R. J. (2007). Developmental cognitive neuroscience: Origins, issues, and prospects. Developmental Review, 27(3), 428-441. [ Links ]
55. Perea, A. E., Alday, L. y Castellano, J. (2006). Registro de datos observacionales a partir del MATCH VISION STUDIO v1.0. En J. Castellano, L. M. Sautu, A. Blanco-Villaseñor, A. Hernández Mendo, A. Goñi y F. Martínez (Eds.), Socialización y Deporte: Revisión crítica (pp. 135-152). Vitoria-Gasteiz: Arabako Foru Aldundia-Diputación Foral de Álava. [ Links ]
56. Posner, M. I., Rothbart, M. K., & Sheese, B. E. (2007). Attention genes. Developmental Science, 10(1), 24-29. [ Links ]
57. Puche, R., & Martí, E. (2011). Metodologías del cambio. Infancia y Aprendizaje, 34(2), 131-139. [ Links ]
58. Rodrigo, M. J. (2010). Donde la psicología evolutiva y la neurociencia se encuentran: ¿un encuentro amenazador u oportuno? Infancia y Aprendizaje, 33(1), 3-17. [ Links ]
59. Rueda, M. R. (2010). Usar el cerebro para comprender el desarrollo de la mente: un comentario al articulo "Donde la psicología evolutiva y la neurociencia se encuentran: un encuentro amenazador u oportuno? de María-José Rodrigo. Infancia y Aprendizaje, 33(1), 35-39. [ Links ]
60. SAS Institute Inc. (2004). SAS 9.1.3 Help and documentation. Cary, NC: SAS Institute Inc. [ Links ]
61. Schlotzhauer, S. D., & Littell, R. C. (1997). SAS system for elementary statistical analysis, Cary, NC: SAS Institute Inc. [ Links ]
62. Schmidt, K. R. T., & Teti, D. M. (2005). Issues in the use of longitudinal and cross-sectional designs. In D. M. Teti (Ed.), Handbook of research methods in Developmental Science (pp. 3-20). Malden, MA: Blackwell Publishing. [ Links ]
63. Siegler, R. S. (2007). Cognitive variability. Developmental Science, 10(1), 104-109. [ Links ]
64. Siegler, R. S., & Crowley, K. (1991). The microgenetic method. A direct means for studying cognitive development. American Psychologist, 46, 606-620. [ Links ]
65. Tau, G. Z., & Peterson, B. D. (2010). Normal development of brain circuits. Neuropsychopharmacology, 35, 147-168. [ Links ]
66. Torbeyns, J., Arnaud, L., Lemaire, P., & Verschaffel, L. (2004). Cognitive change as strategy change. In A. Demetriou and A. Raftopoulos (Eds.), Cognitive developmental change. Theories, models and measurement (pp. 186-216). Cambridge: Cambridge University Press. [ Links ]
67. Vallejo, G., Fernández, P., Livacic-Rojas, P., & Tuero-Herrero, E. (2011). Comparison of modern methods for analyzing unbalanced repeated measures data. Multivariate Behavioral Research, 46, 900-937. [ Links ]
68. van Dijk, M., & van Geert, P. (2011). Heuristic techniques for the analysis of variability as a dynamic aspect of change. Infancia y Aprendizaje, 34(2), 151-167. [ Links ]
69. Walker, S. P., Wachs, T. D., Gardner, J. M., Lozoff, B., Wasserman, G. A., Pollitt, E., Carter, J. A., et al. International Child Development Steering Group. (2007). Child development: risk factors for adverse outcome in developing countries. Lancet, 369(9556), 145-157. [ Links ]
70. West, R., Murphy, K. J., Armilio, M. L., Craik, F. I. M., & Stuss, D. T. (2002). Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition, 49, 402-419. [ Links ]
71. Whelan, T. B., & Mathews, M. J. (2011). A general systems and social-ecological approach to the Neuropsychology of children with neurogenetic disorders. In S. Goldstein and C. R. Reynolds (Eds.), Handbook of neurodevelopmental and genetic disorders in children (pp. 84-101). New York: Guilford Press. [ Links ]
![]() Correspondence:
Correspondence:
Elena Escolano-Pérez.
Facultad de Educación.
Universidad de Zaragoza.
C/ Pedro Cerbuna, 12.
50009 Zaragoza (Spain).
E-mail: eescola@unizar.es
Article received: 20-01-2013;
revised: 10-05-2013;
accepted: 10-03-2014