Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.6 Madrid jun. 2004
| CLINICAL NOTES |
Pyoderma gangrenosum associated with ulcerative colitis: response to infliximab
A. López San Román, F. Bermejo, I. Aldanondo1, E. Carrera, D. Boixeda and E. Muñoz Zato1
Services of Gastroenterology and 1Dermatology. University Hospital Ramón y Cajal. Madrid. Spain
ABSTRACT
Pyoderma gangrenosum is an extraintestinal manifestation of inflammatory bowel disease that can be therapeutically troublesome. We comment on the case of a patient with clinically inactive ulcerat-ive colitis who progressively developed necrotic lesions on both tibial aspects of his legs, which corresponded both clinically and histologically to pyoderma gangrenosum. Treatment with steroids and azathioprine could not control this complication. A single dose of infliximab 5 mg/kg was given, achieving an impressive response of the skin lesions followed by complete healing 3 months later. Infliximab can be useful in the management of refractory extraintestinal manifestations of inflammatory bowel disease.
Key words: Infliximab. Ulcerative colitis. Pyoderma gangrenosum.
López San Román A, Bermejo F, Aldanondo I, Carrera E, Boixeda D, Muñoz Zato E. Pyoderma gangrenosum associated with ulcerative colitis: response to infliximab. Rev Esp Enferm Dig 2004; 96: 420-424.
Recibido: 27-01-04.
Aceptado: 02-02-04.
Correspondencia: Fernando Bermejo San José. Ríos Rosas, 17, 5º C. 28003 Madrid. e-mail: fbermejos@medynet.com
INTRODUCTION
Although the different indications of infliximab therapy in inflammatory bowel disease (IBD) are nowadays better established thanks to clinical trials, there are some circumstances whose not so frequent presentation determines that available evidence appears rather as short clinical series or even isolated clinical cases. A typical example are extraintestinal manifestations, which can condition the therapeutical approach to IBD (1-3). Mucocutaneous manifestations like erythema nodosum and pyoderma gangrenosum (PG) are of special importance. The latter is a specially destruc-tive lesion whose activity not always parallels that of the intestinal disease, and gives way to special therapeutic challenges. We assisted a patient diagnosed with ulcerative colitis who developed a bilateral PG in his legs, refractory to steroids and azathioprine. A complete response was achieved by the off-label use of infliximab.
CASE REPORT
A 45-year-old male had been diagnosed 12 years be-fore with ulcerative pancolitis. While on prophylaxis with mesalazine (1200 mg/d) he consulted in another hospital because of the appearance of bilateral lesions on the anterior aspects of his legs consisting in ulcers with a necrotic center and livid margins, and a destructive tendency; they had been biopsied and identified as PG. His colitis appeared to be clinically inactive, which was confirmed by endoscopy and biopsy. Local cures, accompanied by oral prednisone (1 mg per kg and day), and azathioprine adjusted to erythrocyte thiopurine methyl-transferase activity to 2.5 mg/kg/day. One month later the lesions had not got any better, and therefore he was transferred to our center.
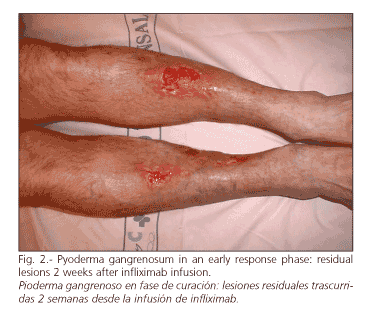
On admission, lesions showed marked ulceration and necrosis, as well as intense inflammatory activity (Fig. 1). Both serum C-reactive protein (7.1 mg/l) and erythrocyte sedimentation rate (26 mm/h) were slightly elevated. After two more weeks of the previous treatment, time during which the absence of active colitis and the histological diagnosis of skin lesions were confirmed, the pyoderma worsened, and the patient was informed about the different possibilities of therapy. The final decision favored an off-label use of infliximab (Remicade®, Centocor, Malvern, PA, USA). Before the drug was used, active infection, positivity of antinuclear antibodies and presence of tuberculous infection were all ruled out, the latter by intradermal PPD (followed by a booster dose one week later), anamnesis and chest X-rays (4). After two months of prednisone and azathioprine therapy, infliximab 5 mg per kg was administered in i.v. infusion, followed by a rapid amelioration of skin lesions (Fig. 2). Complete healing was achieved 3 months later.
The patient has remained under azathioprine mono-therapy. Steroids were withdrawn 3 months after the infliximab perfusion. Maintenance reinfusions have been planned but have not been necessary, since lesions have not recurred after 12 months of follow-up.
DISCUSSION
Extraintestinal manifestations complicate the course of IBD in a significant percentage of cases (1,2). Pyoderma gangrenosum is rare amongst extraintestinal mucocutaneous manifestations and affects 1-5% of patients with IBD (5,6), although in our own experience its incidence has been even lower (0.7% of ulcerative colitis) (7). It is seen more frequently in ulcerative colitis than in Crohn's disease, and initially manifests as painful pustulae that grow into ulcers with livid margins and a necrotic base; lesions can be multiple and leave a scar upon healing. Their preferential location is the anterior aspect of legs (6).
PG can respond to the treatment of IBD, but its relationship to this is less clear than that of other mucocutaneous manifestations such as erythema nodosum (5). Sometimes it has a completely independent course, and, as happened in our case, needs specific treatment. For this, many drugs have been empirically used, like steroids, azathioprine (both useless in our patient), cyclosporine (8) and other drugs.
Infliximab is a monoclonal antibody against tumor necrosis factor-alpha (TNF-α) whose efficiency has been proved in patients with Crohn's disease (9,10). Its possible use in the management of ulcerative colitis is currently subject to investigation (11-13). The response of extraintestinal mucocutaneous manifestations to this drug, has been recently described in clinical cases or short series (14-24), opening new ways to its management. A good response has nearly always been observed. In a recent retrospective series of 13 patients with PG (12 with Crohn's disease and 1 with ulcerative colitis), the efficacy of infliximab reached 100% (25). Administration schedule has been all but uniform in the various published cases. In some of them an initial infusion was used, followed by another in weeks 2 and 6. In many instances additional doses were deemed necessary. We opted for a single infusion in our patient, as in two of the cases studied by Regueiro et al (25). Future studies will have to elucidate which is the ideal schedule in these patients. Recommendations from the Spanish Group for the Study of Crohn's disease and Ulcerative Colitis (GETECCU), point to the convenience of using three doses in such cases, as is currently widespread practice in the management of inflammatory Crohn's disease, a setting where a single dose was earlier recommended (26).
The pathogenesis of PG is far from clear, but this lesion probably involves immune mechanisms akin to those implied in IBD. This is the reason why different therapies aiming at controlling the inflammatory response, like infliximab itself, have been used in its management. A further speculation is whether the positive effect of infliximab is a direct one or is rather mediated by the control of intestinal inflammation (20). Our case, where no intestinal activity was present, seems to support the former option.
Scarce and not uniformly gathered evidence makes it difficult to draw conclusions. Nevertheless, it is clear that infliximab can be a good option to control extraintestinal manifestations that grow into a protagonist clinical role and do not respond to usual therapeutic measures.
REFERENCES
1. Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol 1996; 23: 29-34. [ Links ]
2. Levine JB, Lukawski-Trubish D. Extraintestinal considerations in IBD. Gastroenterol Clin North Am 1995; 24: 633-46. [ Links ]
3. Grand RJ. Treatment of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4: 72-3. [ Links ]
4. Obrador A, López San Román A, Muñoz P, Fortún J, Gassull MA. Consensus guideline on tuberculosis and treatment of inflammatory bowel disease with infliximab. Gastroenterol Hepatol 2003; 26: 29-33. [ Links ]
5. Apgar JT. Newer aspects of inflammatory bowel disease and its cutaneous manifestations: a selective review. Semin Dermatol 1991; 10: 138-47. [ Links ]
6. Callen JP. Pyoderma gangrenosum. Lancet 1998; 351: 581-5. [ Links ]
7. Valer P, Bermejo F, López San Román A, Carrera E, González R, García Plaza A. Extraintestinal manifestations of inflammatory bowel disease (abstract). Rev Esp Enferm Dig 2003; 95 (Supl I): 79. [ Links ]
8. Friedman S, Marion JF, Scheri E, Rubin PH, Present DH. Intravenous cyclosporin in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis 2001; 7: 1-7. [ Links ]
9. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Dis-ease cA2 Study Group. N Engl J Med 1997; 337: 1029-35. [ Links ]
10. Present DH, Rutgeerts P, Targan SR, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340: 1398-405. [ Links ]
11. Su C, Salzberg BA, Lewis JD, Deren J, Kornbluth A, Katzka DA, et al. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol 2002; 97: 2576-84. [ Links ]
12. Probert CSJ, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott IDR, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003; 52: 998-1002. [ Links ]
13. Bermejo F, López San Román A, Hinojosa J, Castro L, Jurado C, Gómez-Belda AB. Infliximab induces clinical, endoscopical and histological response in refractory ulcerative colitis. Rev Esp Enferm Dig 2004; 96: 94-102. [ Links ]
14. Botros N, Pickover L, Das KM. Image of the Month. Pyoderma gangrenosum caused by ulcerative colitis. Gastroenterology 2000; 118: 654. [ Links ]
15. Tan MH, Gordon M, Lebwohl O, George J, Lebwohl MG. Improvement of pyoderma gangrenosum and psoriasis associated with Crohn disease with anti-tumor necrosis factor alpha monoclonal antibody. Arch Dermatol 2001; 137: 930-3. [ Links ]
16. Arnott ID, McDonald D, Williams A, Ghosh S. Clinical use of Infliximab in Crohn's disease: the Edinburgh experience. Aliment Pharmacol Ther. 2001; 15: 1639-46. [ Links ]
17. Hong JJ, Merel NJ, Hanauer SB. Treatment of pyoderma gangrenosum complicating Crohn's disease with infliximab. Gastroenterology 2001; 120: A621. [ Links ]
18. Romero-Gómez M, Sánchez-Muñoz D. Infliximab induces remission of pyoderma gangrenosum. Eur J Gastroenterol Hepatol 2002; 14: 907. [ Links ]
19. Triantafillidis JK, Cheracakis P, Sklavaina M, Apostolopoulou K. Favorable response to infliximab treatment in a patient with active Crohn disease and pyoderma gangrenosum. Scand J Gastroenterol 2002; 37: 863-5. [ Links ]
20. Ljung T, Staun M, Grove O, Fausa O, Vatn MH, Hellstrom PM. Pyoderma gangrenosum associated with Crohn's disease: effect of TNF-alpha blockade with infliximab. Scand J Gastroenterol 2002; 37: 1108-10. [ Links ]
21. Grange F, Djilali-Bouzina F, Weiss AM, Polette A, Guillaume JC. Corticosteroid-resistant pyoderma gangrenosum associated with Crohn's disease: rapid cure with infliximab. Dermatology 2002; 205: 278-80. [ Links ]
22. Batres LA, Mamula P, Baldassano RN. Resolution of severe peristomal pyoderma gangrenosum with infliximab in a child with Crohn's disease. J Pediatr Gastroenterol Nutr 2002; 34: 558-60. [ Links ]
23. Zaccagna A, Bertone A, Puiatti P, Picciotto F, Sprujevnik T, Santucci R, et al. Anti-tumor necrosis factor alpha monoclonal antibody (infliximab) for the treatment of Pyoderma gangrenosum associated with Crohn's disease. Eur J Dermatol 2003; 13: 258-60. [ Links ]
24. Mimouni D, Anhalt GJ, Kouba DJ, Nousari HC. Infliximab for peristomal pyoderma gangrenosum. Br J Dermatol 2003; 148: 813-6. [ Links ]
25. Regueiro M, Valentine J, Plevy S, Fleisher MR, Lichtenstein GR. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol 2003; 98: 1821-6. [ Links ]
26. Domènech E, Esteve-Comas M, Gomollón F, Hinojosa J, Obrador A, Panés J, et al. Recommendations for the use of infliximab (Remicade®) in Crohn's disease. GETECCU 2001. Gastroenterol Hepatol 2002; 25: 162-9. [ Links ]











 texto en
texto en