Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.2 Madrid feb. 2005
| ORIGINAL PAPERS |
Prevalence of silent fecal and urinary incontinence in women from the town of Teruel
A. Ballester, M. Mínguez1, B. Herreros1, V. Hernández1, V. Sanchiz1 and A. Benages1
Familiar and Communitary Medicine. Centro de Salud de Catarroja. 1Department of Gastroenterology. Hospital Clínico Universitario. University of Valencia. Valencia, Spain
ABSTRACT
Objectives: to study the prevalence of fecal (FI) and urinary incontinence (UI) in women from Teruel (Spain), as well as the clinical conditions associated with these disorders.
Methods: we studied prospectively women with an age range of 20-64 yrs. who were randomly selected from the population seen in a primary care center because of medical disorders not related to incontinence. Patients with functional or cognitive impairment were excluded. Medical and obstetric antecedents, as well as the type and frequency of incontinence symptoms were collected in a questionnaire.
Results: out of 115 women, 103 completed the study (mean age: 41±12 yrs. range 20-64). UI was present in 34.9% (stress 33%, urge 14%, mixed 47%), FI in 14 (13.6%) (flatus 57%, liquid stools 43%), and 10 (9.7%) displayed both disorders. Age > 42 yr. and body mass index ≥ 25 were associated with FI and UI; pregnancy was only associated with UI, but the group of women with ≥ 2 vaginal deliveries showed a higher frequency of FI (p < 0.05, Chi squared test). In the multivariate analysis, only the presence of UI was associated with FI (OR 6.0; CI 95% 1.7-21). Association of FI and UI was more frequent in women older than 42 yr. (OR 16.7, CI 95% 1.9-141). No statistical differences were found when smoking, exercise, and type of childbirth were compared between the presence/absence of FI or UI.
Conclusions: urinary and fecal incontinence are frequent in women, and the coexistence of both disorders is not uncommon. Age, overweight and parity are associated with the presence of fecal and/or urinary incontinence.
Key words: Fecal incontinence. Urinary incontinence. Women. Prevalence. Risk factors.
Ballester A, Mínguez M, Herreros B, Hernández V, Sanchiz V, Benages A. Prevalence of silent fecal and urinary incontinence in women from the town of Teruel. Rev Esp Enferm Dig 2003; 95: 78-86.
Recibido: 18-06-04.
Aceptado: 19-10-04.
Correspondencia: A. Benages. Servicio de Gastroenterología. Hospital Clínico Universitario de Valencia. Avd.a Blsco Ibáñez, 17. 46010 Valencia. Fax: 963864767 - e-mail: benages@uv.es
Partly supported by a grant from Instituto de Salud Carlos III (03/02).
INTRODUCTION
Fecal incontinence (FI) and urinary incontinence (UI) are distressing conditions with great social and economic impact. FI affects 2.2-15.3% of the population (1-6), especially women, and its prevalence is known to increase with age and in specific groups such institutionalized patients, neurologic disorders (spina bifida, multiple sclerosis), diabetes mellitus, or irritable bowel syndrome (6). The prevalence of FI is unknown because patients usually hide this symptom (up to 64.7% of women in some countries) (4). It has been reported that a third of patients with FI do not complain of that (7), and less than half of women with diarrhea associated with incontinence report this symptom (8).
UI affects 50-65% of institutionalized patients, and most of them have double incontinence (9-12). Discrepancies between data from epidemiological studies about UI are related to a lack of unified definition criteria for UI, collection methods, and measurement of symptoms (13). Moreover, few studies have studied the coexistence of FI and UI, and factors related to this disorder in women (14,15). Double incontinence has been recently analyzed in a Spanish study, which showed a prevalence rate of 8.7% among women with UI (15).
The aim of this study was to identify FI and UI in women from the town of Teruel who visited a primary care center because of medical disorders not related to incontinence, as well as factors associated with incontinence. We excluded patients aged more than 65 yr. and the presence of well-known risk factors for incontinence (13), functional and cognitive impairment, and deterioration of the general health status due to systemic diseases.
MATERIAL AND METHODS
A prospective study was conducted in the only primary care center of Teruel between October 2002 and May 2003. Demographic and clinical data were registered in a questionnaire that included symptoms of fecal and urinary incontinence, medical and surgical antecedents, obstetric history (number of births, weight of neonates, type of delivery, instrumental maneuvers, and pelvic floor injury at delivery), smoking, consumption of alcohol, exercise, and body mass index (BMI).
Subjective criteria were used to assess UI, including subtypes of UI (stress, urge, mixed, involuntary), frequency (never; less than one episode per month; more than one episode per month but less than one per week; one or more episodes per week but less than one per day; one or more episodes per day), and quality of life status.
FI was evaluated according to the severity score by Jorge and Wexner (16).
Subjects
Selection was randomly applied to the first woman seen every day (from Tuesday to Friday every week) in the primary care center who met inclusion/exclusion criteria. They underwent a clinical evaluation by means of a questionnaire filled out by a doctor, and inclusion criteria included: age range of 20-64 years; voluntary acceptance to participate in the study; reason for consultation other than incontinence; adequate cognitive status to understand the questionnaire. We excluded institutionalized patients as well as those with conditions entailing physical, neurologic or cognitive impairment.
Size sample
The only primary care center in Teruel provides medical care to the whole of its population. Out of 16,255 women, 9,316 with an age range of 20-64 years made up for 57.31% of the female group. Based on these data, a sample of 1% of all women in this age group was estimated for the study.
Statistical analysis
Study groups were established according to the presence or absence of incontinence (fecal, urinary or both), and differences between groups, based on data from the questionnaire, were statistically analyzed using the Chi squared test for qualitative parameters and Student's t-test for quantitative ones. A multivariate analysis was performed to analyze predictive factors related to incontinence (Wald method). Data are expressed as mean ± SD. The double incontinence group includes patients with anal plus urinary incontinence. A value of "p" less than 0.05 was regarded as statistically significant.
RESULTS
Description of the sample
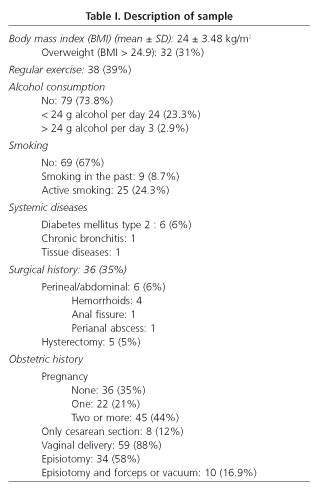
Out of 115 women evaluated, 103 (89.5%) fulfilled the inclusion criteria and completed the evaluation (mean age: 41.67 ± 12.09 yr.; median: 42; range 20-64). The study group comprised 1.1% of all women in this age range. Demographic and clinical features are shown in table I.
Urinary incontinence
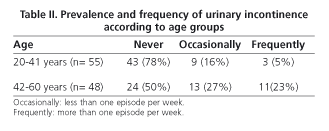
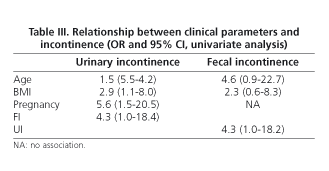
According to data from the questionnaire, 36 women (34.9%) reported UI [stress 12 (33%), urge 5 (14%), mixed 17 (47%) and involuntary 2 (5%)]. Only 27.7% of incontinent women used urinary pads regularly. Leakage of urine caused social limitation in 22.2% of women and affected quality of life in 33%. The prevalence of UI based on stratified age is shown in table II. Compared with continent women, age was higher in the group of women with UI (38 ± 12 vs. 47 ± 10 yr., p < 0.001, Student's t-test), as well as BMI (23.3 ± 3 vs. 25.2 ± 4, p < 0.05, Student's t-test). Among women with UI, 27.7% had also FI. Univariate analysis showed that UI was significantly associated with age > 42 yr., overweight, parity history (vaginal delivery and/or cesarean section), and presence of FI (Table III). However, only overweight (OR 2.97, CI 1.1-7.9), parity (OR 6.8, CI 2.0-23.0) and FI (OR 4.8, IC 1.1-20.1) were related to UI in the multivariate analysis. No association with the presence of UI was found when delivery, type of delivery (vaginal delivery/cesarean section), weight of neonates, smoking, and exercise were analyzed.
Two women used anal pads regularly, and only two reported affectation of quality of life due to FI (occasionally). The mean value of severity score of FI was 1.5 ± 1.16 (1 point in eleven women, 2 points in one, 3 points in one, and 5 points in another one).
Age in women with FI was older than in continent women (51.14 ± 13.67 yr. vs. 40.18 ± 11.19 yr., p = 0.001, Student's t-test). No differences between types of FI (flatus/liquid stools) and age were detected. The presence of FI was associated with UI: 10/14 women with FI showed UI (71.42%) compared with only 26/89 without FI (29.21%) (p = 0.002, Chi squared test).
No statistical association was observed between the presence of FI and smoking, consumption of alcohol, exercise, and medical or surgical history.
Only 3/36 nullipara women reported FI (flatus: 2, liquid stools: 1) whereas 11/67 women with previous delivery (16.41%) showed it (p = 0.36, Chi squared test).
Type of delivery (vaginal vs. cesarean section) did not influence the presence of FI, but it is interesting to note that none of the women with a history of cesarean section showed FI, whereas 11/59 women with a history of vaginal delivery (18.64%) did. The number of vaginal deliveries was related to the presence of FI (one: 4.54%; two: 25.92%; three or more 30%, p = 0.047, Chi squared test). The weight of neonates did not correlate with the presence of FI in women with vaginal delivery (3365 ± 0.503 g in continent women vs. 3523 ± 0.696 g in women with FI, p = 0.413, Student's t test) or antecedents of instrumental maneuvers during vaginal delivery (FI in 4/15 (26.6%) of women without instrumentation, 6/34 (17.6%) with episiotomy, and 1/10 (10%) with forceps or vacuum (p = 0.28, Chi squared test).
According to the univariate analysis, FI was associated with age > 42 yr., overweight, and presence of UI (Table III); however, only UI was related to FI in the multivariate analysis (OR 6.0, CI 1.7-21.0).
Fecal and urinary incontinence
Ten women (9.7%) had both FI and UI. Compared with continent women, they showed older age (52.6 ± 12.5 yr. vs. 38.1 ± 11.4 yr., p < 0.001, Student's t test) and a higher number of births (2.3 ± 0.5 vs. 1.6 ± 0.5, p < 0.04, Student's t test), but no differences were detected when the number of vaginal deliveries was analyzed. None of the nullipara women showed double incontinence. Only age > 42 yr. was related to the presence of double incontinence in the multivariate analysis (OR 16.7, CI 1.9-141.1).
DISCUSSION
The etiology of incontinence is multifactorial, and factors such as age, gender, systemic or psychiatric diseases, immobility, and physical or cognitive impairment may contribute to its development. Selected patients with these conditions accumulate the greater rates of incontinence in epidemiological studies, and it is difficult to identify other factors associated with incontinence in these groups. In order to establish a more restrictive sample, we randomly selected women from the population aged 20-64 yr. who attended a primary care center for reasons other than incontinence, and we easily ruled out, by means of a well-structured questionnaire, conditions apt to increase the prevalence of incontinence.
Although the sample size included in our study is representative of the population of Teruel (more than 1%), we think that it could be insufficient to detect statistical differences when differentiating subtypes of incontinence. In accord with most epidemiological studies, we did not employ objective criteria for evaluating the presence of UI according to the criteria of the International Continence Society ("involuntary loss of urine that is a social or hygienic concern and is objectively demonstrable") (17), since demonstrable leakage is difficult to establish.
A comparison of available data on the prevalence of incontinence is difficult because of variability in the selection criteria of patients (age, gender, comorbility) and methods of study. In our series including socially active women without functional or cognitive impairment, we detected a prevalence rate of UI of 34.9%, which is similar to that of other series (10-40%) (18-21) including women of older age. UI increases with age, as has been reported widely in previous reports. We detected a lower prevalence of UI in women < 42 yr. (21%) versus those with ages between 42 and 64 years (50%), as shown by Minassian et al. (22) in a study that analyzed the prevalence of UI in women included in 13 epidemiological studies from around the world and whose age was stratified into decades. In Spain, Gavira Iglesias et al. (23) reported a prevalence of UI of 42% in women older than 65 years.
Similarly to other authors, we did not detect any association between incontinence and smoking or exercise (22).
Overweight has been detected as a risk factor for UI in both sexes in large population-based studies (22); our results indicate that overweight is related to both UI and FI. This finding was described too by Fornell et al. in a group of women (24), and it may be explained from the injury of pelvic floor muscles due to the higher intra-abdominal pressures induced by overweight (25).
According to our results, a history of childbirth is a risk factor for UI (OR 6.8), but not for FI. Type of delivery is not related to FI or UI, in accord with others reports (5). However, the number of vaginal deliveries is significantly associated with the presence of FI, since its prevalence in women with two or more vaginal deliveries was 27%, whereas FI was present in 4.5% of women with only one. This finding, widely reported in the literature, (1,5,22,24,26-28), should be carefully analyzed because the presence of other frequent factors related to incontinence (age, physical or cognitive limitations) may over-estimate the prevalence of FI attributed to vaginal delivery (1,5). In our study, although age is associated with FI (OR 4.6), the presence of UI is the most important factor related to FI in the multivariate analysis (OR 6). Although we did not detect statistical differences between a history of maneuvers during vaginal delivery (episiotomy, forceps, etc.) and the presence of FI or UI, we think that our sample size is not enough to assess the impact of these maneuvers. According to the Wexner score, the severity of FI was low in our series (mean of 1.5 points, range: 1 point (eleven women) and 5 points (one women); considering a maximum score of 20, we observed that women with FI had a low severity, which may explain the infrequent affectation of quality of life by this disorder (only two women and rarely, less than once per month).
The prevalence of double incontinence in our study (9.7%) is similar to that in other studies that included women of 18-49 yr. (8%) (21), and in another one from Spain that studied prevalence in a group of women with UI (8.7%) (15). In accord with our results, age is the most important factor related to the presence of double incontinence (OR 16.7), and the number of births was significantly higher in this group. These findings, in accord with other reports, suggest that age is the most relevant factor for the weakness of pelvic floor muscles in women.
The impairment of quality of life from UI has been assessed by means of two simple questions: social limitation and changes in lifestyle. In women with UI, 22.2% had social limitations and 33% reported an affected lifestyle (rarely). Studies about quality of life in FI show different degrees of affectation in 33-34% of patients of both genders (19,23).
REFERENCES
1. Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA 1995; 274: 559-61. [ Links ]
2. Reilly W, Talley N, Pemberton J. Fecal incontinence: prevalence and risk factors in the community. Gastroenterology 1995; 108: A32. [ Links ]
3. Walter S, Hallbook O, Gotthard R, Bergmark M, Sjodahl R. A population-based study on bowel habits in Swedish community: prevalence of faecal incontinence and constipation. Scand J Gastroenterol 2002; 8: 911-6. [ Links ]
4. Ritz DE, Hassan MY, Shaheen H, Cherian JV, Micallef R, Dunn E. The prevalence and determinants of health care-seeking behavior for fecal incontinence in multiparous United Arab Emirates females. Dis Colon Rectum 2001; 12: 1850-6. [ Links ]
5. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000; 12: 1460-70. [ Links ]
6. Whitehead W, Wald A, Norton N. Treatment options for fecal incontinence. Dis Colon Rectum 2001; 44: 131-44. [ Links ]
7. Johanson JF, Lafferty J. Epidemiology of fecal incontinence:the silent affiction. Am J Gastroenterol 1996; 91: 33-6. [ Links ]
8. Leigh RJ, Turnberg LA. Faecal incontinence: the unvoiced symptom. Lancet 1982; I : 1349-51. [ Links ]
9. Harrington C, Carrillo H, Thollaug SC, Summers PR, Wellin V. Nursing facilities, staffing, residents, and facility deficiencies, 1993 through 1999. Departament of social and behavioural Sciences. University of California, San Francisco, CA October 2000. [ Links ]
10. Dollard KJ. Facts and trends. American Health Care Association´s Research and Information Services Group. American Health Care Association, 1997. [ Links ]
11. Johanson JF, Irizarry F, Doughty A. Risk factors for faecal incontinence in a nursing home population. J Clin Gastroenterol 1997; 24: 156-60. [ Links ]
12. Chiang L, Ouslander J, Schelle JF, Reuben DB. Dually incontinent nursing home residents: clinical characteristics and treatment differences. J Am Geriatr Soc 2000; 48: 673-6. [ Links ]
13. Espuña M. Incontinencia de orina en la mujer. Diagnóstico y tratamiento. Med Clin (Barc.) 2003; 120: 464-72. [ Links ]
14. Lacima G, Pera M. Combined fecal and urinay incontinence an update. Current Opinion in Obstetrics Gynecology 2003: 15: 405-10. [ Links ]
15. Lacima G, Espuña M, Pera M, Puig-Clota M, Quinto L, García-Valdecasas JC. Clinical, urodynamic, and manometric findings in women with combined fecal and urinary incontinence. Neurourol Urodyn 2002; 21: 464-9. [ Links ]
16. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993; 36: 77-97. [ Links ]
17. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinay tract function. Report from the Standardisation Sub-committee of the International Continence Society. Neurourol and Urodyn 2002; 21: 167-78. [ Links ]
18. Herzog AR, Fultz NH. Prevalence and incidence of urinary incontinence in community dwelling populations. J Am Geriatric Soc 1990; 38: 273-81. [ Links ]
19. Hannestad YS, Rortveit G, Sanvick H, Hunskar S. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT Study. J Clin Epidemiol 2000; 53: 1150-7. [ Links ]
20. Dolan LM Casson K, Mcdonald P, Ashe RG. Urinary incontinence in Northen Ireland: a prevalence study. BJU Int 1999; 83: 760-6. [ Links ]
21. Siracusano S, Pregazzi R, d´Aloia G, Sartore A, Di Benedetto P, Pecorari V, et al. Prevalence of urinary incontinence in young and middle-aged women in an Italian urban area. Eur J Obstet Gynecol Reprod Biol 2003; 107: 201-4. [ Links ]
22. Minassian VA, Drutz HP, AL-Badr A. Urinary incontinence as a worldwide problem. Int J Gynecol Obstet 2003; 82: 327-38. [ Links ]
23. Gavira Iglesias FJ, Caridad y Ocerin JM, Pérez del Molino Martín J, Valderrama Gama E, López Pérez M, Romero López M, et al. Prevalence and psychosocial impact of urinary incontinence in older people of a Spanish rural population. J Gerontol A Biol Sci Med Sci 2000: 55; 207-14. [ Links ]
24. Fornell EA, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand 2004; 83: 383-9. [ Links ]
25. Noblett KL, Ostergard DR. The relationship of body mass index to intra-abdominal pressure as measured by multichannel cystometry. Int Urogynecol J Pelvic Floor Dysfunct 1997; 8: 323-6. [ Links ]
26. Nelson RL. Epidemiology of fecal incontinence: Dimensions of the problem: prevalence and impact. Gastroenterology 2004; 126: S3-S7. [ Links ]
27. Small KA, Wynne JM. Evaluating the pelvic floor in obstetric patients. Aust N Z Obstet Gynecol 1990; 30: 41-5. [ Links ]
28. Madoff RD, Williams JG, Caushaj PF. Current concepts: fecal incontinence. N Engl J Med 1992; 336: 1002-7. [ Links ]











 texto en
texto en