Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.7 Madrid jul. 2005
| ORIGINAL PAPERS |
Maintenance of T1 response as induced during PEG-IFNa plus ribavirin therapy
controls viral replication in genotype-1 patients with chronic hepatitis C
M. Trapero, L. García-Buey, C. Muñoz1, M. Vitón1, J. A. Moreno-Monteagudo, M. J. Borque2, N. E. Quintana1 and R. Moreno-Otero
Unit of Hepatology. 1Service of Immunology. 2Unit of Molecular Biology. Hospital Universitario de La Princesa. Universidad Autónoma.
Madrid, Spain
ABSTRACT
Objectives: to analyze the T1/T2 cytokine profile in CD8 T cells from peripheral blood mononuclear cells from patients with genotype-1 CHC during treatment with pegylated interferon (Peg-IFN) α2a plus ribavirin (RBV). To correlate Th1/Th2 balance with virological response.
Patients and methods: in this prospective longitudinal study, a total of 28 naïve genotype-1 CHC patients received Peg-IFNα2a (180 µg/week) plus RBV (1-1.2 g/day) for 48 weeks. All patients (mean age 45 ± 8 years) completed treatment and follow-up: 12 (43%) achieved a sustained virological response (SVR), 13 relapsed after end of treatment (47%), and only 3 (10%) were non-responders. Sixteen healthy controls were also analyzed (mean age 39 ± 17 years). The production of IL-4, IIFNγ, and TTNFα by CD8 T cells was measured by intracytoplasmic detection using flow cytometry in both resting and stimulated cells with a phorbol ester. Statistics: Student's t test for independent values, χ2 test, and ANOVA test were used; relapsers and non-responders were joined to achieve a higher statistical power.
Results: at third month during treatment, phorbol ester-stimulated-IL-4 levels tend to be lower in patients who presented with SVR versus those who did not (0.97 vs 2.58; p = 0.1). No statistically significant differences were found in IIFNγ and TTNFα levels at month 3. At EOT, the stimulated-IIFNγ production was significantly higher in patients with SVR (20 vs. 8; p < 0.05). Conversely, IL-4 production was higher in NR patients although these data did not reach statistical significance (p < 0.1). No significant differences were found in TTNFα (14 vs. 7; p < 0.2).
Conclusions: Cytokine T1 induced-response maintenance during combination treatment, measured as IIFNg production by CD8+ T lymphocytes, is associated with SVR and suggests the replication control and later clearance of patients infected by genotype-1 HCV.
Key words: Hepatitis C virus. Chronic hepatitis. Pegylated interferon. Ribavirin. Cytokines. Lymphocytes.
Trapero M, García-Buey L, Muñoz C, Vitón M, Moreno-Monteagudo AJ, Borque MJ, Quintana NE, Moreno-Otero R. Maintenance of T1 response as induced during PEG-IFNa plus ribavirin therapy controls viral replication in genotype-1 patients with chronic hepatitis C. Rev Esp Enferm Dig 2005; 97: 481-490.
Recibido: 29-11-04.
Aceptado: 08-02-04.
Trabajo aceptado: Ricardo Moreno Otero. Servicio de Aparato Digestivo. Hospital Universitario de La Princesa. C/Diego de León, 62, planta 3ª. 28006 Madrid. e-mail: mtraperomarugan@terra.es; rmoreno.hlpr@salud.madrid.org
INTRODUCTION
Hepatitis C virus (HCV) infection is characterized by a persistence of viral replication, a chronic pattern of disease, and in some patients a progression toward cirrhosis and hepatocellular carcinoma. Mechanisms of cellular damage and viral persistence are not well established, although viral factors and immune response implications are well known (1).
Some virological factors such as viremia, genotype and quasiespecies may play an aggressive role. Nevertheless, host immune response is the most important key in the pathogenesis, principally in viral replication control and hepatocellular damage. HCV-specific CD4+ and CD8+ T cells have been demonstrated in the peripheral blood and liver tissue of patients with chronic hepatitis C (CHC). Cytokine production by these T cells may play an important role in the pathogenesis of CHC, and also has a great impact on viral replication control (1,2).
CD4+ T cell Th1 and Th2 subsets exert distinct actions by secreting different kinds of cytokines. Th1 cells produce IL-2, IIFNγ and TTNFα favoring a cellular-mediated response, while Th2 cells secrete IL-4, IL-5 and IL-10, thus stimulating humoral immunity. It has been demonstrated that a polarization of the immune response towards one or these types may control or maintain infectious disease. Besides, CD8+ T cells, which have been less studied, have T1 and T2 functions, secreting IIFNγ and IL-4 respectively (3,4). CHC patients have a Th1 cytokine profile in liver tissue (5-7) as well as in peripheral blood according to some authors (8,9); nevertheless, other studies have shown a predominant Th2 profile or even no differences at all (10,11).
The modulation of peripheral blood cytokine and intrahepatic cytokine profiles by a combination treatment with PEG-IFNaα plus ribavirin is not well known. Few studies exist, and only patients treated with interferon alone or in combination with ribavirin have been evaluated; however, no data regarding PEG-IFNaα are presently available. Some studies detected a Th2-cytokine decrease (IL-4, IL-10) or a Th1-cytokine increase (IL-2) during treatment with IFN (12,13). Regarding the role of cytokine profile as a predictive factor for response, studies are few and results are inconclusive (14-16).
The function of CD8+ T cells has been scarcely evaluated. Some studies exist related to T cell activation molecules in liver tissue (8-11), but none of them analyzes levels and possible effector functions in peripheral blood mononuclear cells (PBMC).
The aim of this study was to analyze the profile of T1/T2 cytokines produced by peripheral blood CD8+ T cells in CHC patients infected by genotype 1 during treatment with PEG-IFNα2a plus RBV versus that of healthy controls. A second endpoint was to correlate T1/T2 cytokine ratio as produced by CD8+ T cells with virological response to treatment.
PATIENTS AND METHODS
In this prospective, longitudinal study 28 naïve CHC patients with genotype 1 were consecutively included. All of them had elevated serum alanine aminotransferase (ALT) levels, at least twice the upper normal limit, and serum positivity for anti-hepatitis C virus (anti-HCV) by a second-generation immunoenzyme assay (ELISA). HCV RNA was detected by polymerase chain reaction (PCR). Other chronic liver diseases (hereditary, metabolic, toxic or other viruses) were excluded, as were patients with human immunodeficiency virus (HIV) coinfection. A total of 16 healthy controls (M/F = 9/7; 39 ± 17 years) with normal liver function were studied. Physicians treating patients were blinded with respect to the cytokine profile assay. No liver biopsy was performed because of patient refusal. An informed consent was obtained for all participants.
Parameters analyzed included: age, gender, platelets, serum alanine -ALT- and aspartate -AST- aminotransferases, alkaline phosphatase (ALP), γ glutamyl-transpeptidase (GGT) and GGT/ALT ratio. Qualitative and quantitative viremias were tested by using HCV-Amplicor® and HCV-Monitor® (Roche Diagnostic System, Basel, Switzerland), with a sensitivity threshold of 102 (Amplicor®) and 103 (Monitor®) copies/genome equivalent per mL. HCV genotyping was performed by a reverse-hybridization line probe assay (INNO-LIPA HCV; Innogenetics, Zwijndrecht, Belgium).
Periodic determinations of all values at 1, 3, 6, 9 and 12 months during treatment and at 3 and 6 months during follow-up were performed.
CD8+ T cell-secreted cytokine assays
Blood samples were collected in heparin tubes. For T cell stimulation, 25 ng/mL of phorbol 12-myristate13 acetate (PMA) plus 1 μg/mL of ionomycine (calcium ionophor) was added immediately during 4 hours at 37 ºC and 7% CO2, with the subsequent addition of brefeldin-A (Sigma Chemical Co. (St. Louis, Missouri, USA). The production of IL-4, IFNγ and TNFα by CD8+ T cells from PBMC was measured by intracytoplasmic detection using flow cytometry (FACScan fluorescent cell sorter, Becton Dickinson and Company). These values were obtained in T cells both at rest and under stimulation with PMA. First, a surface staining of PBMC was performed with monoclonal antibodies: antiCD4 conjugated with fluorescein isotiocianate (FITC) and antiCD8 conjugated with phycoeritrin (PE). T lymphocytes with anti-CD3 PE and anti-CD69 FITC were used as stimulation controls. T cell permeabilization was then performed using the lysing solution kit according to the manufacturer's instructions.
For T cell intracellular staining, monoclonal antibodies were also utilized: anti-IFNγ conjugated with FITC, anti-IL-4 conjugated with PE, and anti-TNFα conjugated with FITC. All of them were commercialized by Becton Dickinson (San José, California, USA) (14).
All cytokines were measured in healthy controls and in CHC patients; in the latter before treatment (month 0), during treatment (months 1, 3, 6, 9 and 12) and during follow-up (months 15 and 18). Cytokine levels are expressed as percentage of CD8+ T cells from peripheral blood that express each cytokine.
Treatment
All patients received combination treatment with PEG-IFNa-2α (PEGASYS®, Roche), 180 μg weekly subcutaneously, plus ribavirin (COPEGUS®, Roche) orally (1-1.2 g/day), adjusted to body weight (above or below 75 kg) for 48 weeks. This treatment was approved by the ethical committee in our hospital. An informed consent was obtained from all patients.
At third month of treatment early virological response (EVR) was evaluated. The end-of-treatment (EOT) response was defined as HCV RNA clearance and normalization of alanine aminotransferases serum levels on completion of combined treatment at week 48. A sustained virological response (SVR) was defined when this response persisted during 6 months of follow-up (week 72). Non-responders were considered as patients without an SVR, including relapsers (patients with EOT response but without SVR) and patients who achieved no EOT response. All data were analyzed by comparing both groups.
Statistical analysis
Quantitative values are expressed as mean ± standard deviation (SD). Qualitative values as percentage. All possible differences between both groups were analyzed using the Mann-Whitney test, Fisher's test or the χ2 test. A value of p < 0.05 was considered statistically significant. Relapsers and non-repsonders (NR) were pooled together in order to improve the statistical power of the study.
RESULTS
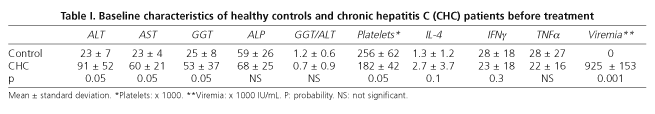
Healthy controls (mean age 39 ± 17 years) were similar to CHC patients regarding age and gender distribution. Platelet levels were higher in healthy controls (256,000 ± 62,000 vs. 182,000 ± 42,000; p < 0.05) and ALT, AST, GGT and ALP levels were normal. No statistically significant differences in resting and stimulated IL-4, IIFNγ and TTNFα production by CD8+ T cells were found (Table I).
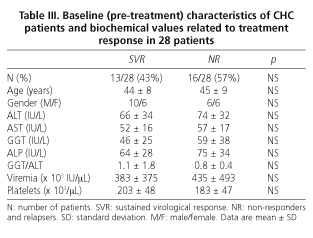
All 28 patients (mean age 45 ± 8 years) finalized the 48-week treatment and 6-month follow-up periods. At the end of this period (72 weeks), 12 of 28 patients achieved an SVR (43%), 13 relapsed (47%), and 3 (10%) were NR. Relapsers and NR were analyzed together in order to improve the statistical power of the study.
Comparison between pre-treatment status and post-follow-up status of patients
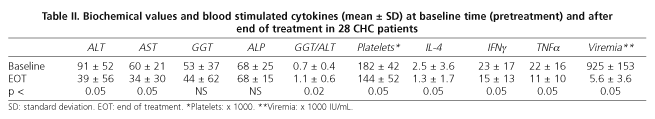
Baseline (pre-treatment) and end-of-treatment characteristics of CHC patients are summarized in table II. As shown in table III, the baseline characteristics of patients achieving SVR are homogeneous and similar to those of non responders (including relapsers), and thus comparable. There were no withdrawals, and it must be emphasized that all CHC patients were naïve and infected by genotype-1 HCV.
Cytokine profile during antiviral treatment
No differences in resting and stimulated IL-4, IFNγ and TNFα levels as produced by peripheral blood CD8+ T cells were found between healthy controls and CHC patients (Table I). Nevertheless, the mean value of stimulated IL-4 tended to be lower in healthy controls than in CHC patients (1.3 ± 1.2 vs. 2.7 ± 3.7; p < 0.1), and the mean of stimulated IIFNγ tended to be higher in healthy controls than in CHC patients (28 ± 18 vs. 23 ± 18; p < 0.3).
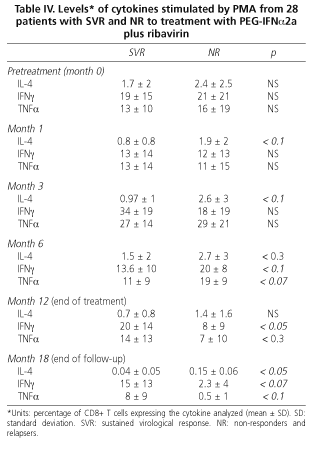
During combination treatment with PEG-IFNα2a plus ribavirin the intracytoplasmic production of IL-4, IFNγ and TNFα decreased irregularly in all patients. At one month no statistically significant differences between responders and non responders were found, although stimulated IL-4 levels tended to be lower in patients with SVR than in NR (0.8 ± 0.8 vs. 1.9 ± 2; p < 0.1). At month 3 of treatment, stimulated IL-4 levels were lower in NR patients, but without statistical significance (0.97 ± 1 vs. 2.58 ± 3; p = 0.1). No differences in IIFNγ or TTNFα levels between patients with SVR or NR were found. At six months of treatment there were no differences regarding cytokine profiles, either at rest or stimulated by PMA, between SVR and NR patients.
At EOT (week 48) the production of stimulated IFNγ was higher in patients who had achieved an SVR (20 ± 14 vs. 8 ± 8; p < 0.05). In contrast, IL-4 production was higher in non-responders, although these data did not reach statistical significance (p < 0.1). No differences in TTNFα production (14 ± 13 vs. 7 ± 10; p < 0.3) were found. At the end of follow up (6 months after therapy completion) patients with SVR maintained higher IIFNγ levels than NR (at the limit of statistical significance), and IL-4 levels were lower in SVR patients than in NR (0.04 ± 0.05 vs. 0.15 ± 0.06; p < 0.05). Regarding TTNFα levels, no differences were found.
DISCUSSION
Immune response against HCV is complex using both cellular and humoral pathways to eradicate the virus. Interaction between different cellular subsets within the immune system -B cells, T cells and other accessory cells- inhibit viral replication; at the same time, this response may be responsible for histological damage (17). A disbalance between T cell subsets and natural killer (NK) cells has been demonstrated in peripheral blood samples from patients with chronic hepatitis C (18-21). The activation of specific T cells and B cells is fundamental for HCV elimination, but not effective in many cases. Besides, this disbalance may result in severe damage in host cells and extend HCV persistence (16). Studies have been carried out to dilucidate whether a proliferative response to a mytogen from PBMC is related to HCV eradication (17).
Other authors have studied the implication of immune cells and different cytokines in the response to antiviral treatment, mainly focusing on CD4+ T cells (15-17). CD8+ T cells show a cytolitic function on recognizing viral antigens bound to class I-HLA molecules. It is well known that these cells play a determinant role in the control of viral infection (19), and intrahepatic cytolitic activity is attributed to activated intrahepatic CD8+ T cells. Besides, they can synthesize and secrete different cytokines such as IFNγ, IL-4 and αTNFα, which modulate other lymphocyte subsets including CD4+ and CD8+ phenotypes (22-24). There were no conclusive studies analyzing the production of these cytokines by CD8+ T cells in peripheral blood samples during treatment with PEG-IFNa plus ribavirin (16). Both PEG-IFNα and ribavirin in combination have antiviral and immunomodulatory effects, favoring a vigorous immune response that may eliminate HCV infection.
In the present study we evaluated the T1/T2 cytokine peripheral blood profile as secreted by CD8+ T cells during combination treatment with PEG-IFNα plus ribavirin in naïve CHC patients with genotype 1. In addition, we correlated these parameters with patterns of response to antiviral treatment, trying to establish possible predictive factors of response. Our study findings suggest that patients with SVR have a CD8+ T cell immune response polarization towards type-1 cytokines (with functional similarities to the Th1 response of CD4+ T cells), with higher levels of IFNγ at end of treatment and during follow up. On the contrary, patients who did not achieve an SVR had a predominant type-2 immune response (similar to the Th2 type of CD4+ T cells) characterized by higher peripheral blood levels of IL-4 at end of treatment, with a subsequent decrease during follow up.
Combination treatment with PEG-IFNα plus ribavirin has a well known immunomodulatory and antiviral action, although its definite mechanisms are not well established (25). Findings from this study suggest that the response to treatment with PEG-IFNα plus ribavirin is partially determined by the proliferation and subsequent activation of the CD8+ lymphocyte subset, with the resulting synthesis of cytokines -T1 predominance would be associated with SVR, whereas a T2 response would not be effective to eliminate HCV and would perpetuate chronic infection.
In summary, the maintenance of cytokine T1-induced response during combination treatment, measured as IFNγ production by CD8+ T lymphocytes, is associated with SVR and suggests replication control and later clearance of patients infected by genotype-1 HCV. Due to the small number of patients and the characteristics of this disease, further studies with a higher number of patients would be necessary to corroborate these results. In addition, the study of other lymphocyte subsets would allow a delimitation of interactions between the various functions of T cell subsets in the overall immune response of patients with chronic hepatitis C.
ACKNOWLEDGEMENTS
Study partially supported by grants to R.M.O. from Instituto de Salud Carlos III (C03/02) and Ministerio de Salud y Tecnología (SAF 2001-1414).
REFERENCES
1. Kamal SM, Graham CS, Bianchi L, Tawil AA, Rasenack JW, Khalifa KA. Kinetics of intrahepatic hepatitis C virus (HCV) - specific CD4+ T cell responses. J Infect Dis 2004; 189: 1140-50. [ Links ]
2. Spengler U, Lechmann M, Irgang B, Dumolin FL, Sauerbruch T. Immune responses in hepatitis C virus infection. J Hepatol 1996; 24 (Supl. 2): 20-5. [ Links ]
3. Koziel MJ. The role of immune responses in the pathogenesis of hepatitis C. J Viral Hepat 1997; 4 (Supl. 2): 31-41. [ Links ]
4. Torres C, Muñoz de Rueda P, Ruiz-Extremera A, Quintero D, Palacios A, Salmerón J. Genomic and antigenomic chains of hepatitis C virus and hepatitis G virus in serum, liver and peripheral blood mononuclear cells. Rev Esp Enferm Dig 2002; 94: 659-68. [ Links ]
5. Shapiro S, Gershtein V, Elias N, Zuckerman E, Salman N, Lahat N. mRNA cytokine profile in peripheral blood cells from chronic hepatitis C virus (HCV)-infected patients: effects of interferon-alpha (IFN-alpha) treatment. Clin Exp Immunol 1998; 114: 55-60. [ Links ]
6. Schlaak JF, Pitz T, Lohr HF, Meyer zum Buschenfelde KH, Gerken G. Interleukin 12 enhances deficient HCV-antigen-induced Th1-type immune response of peripheral blood mononuclear cells. J Med Virol 1998; 56: 112-7. [ Links ]
7. Reiser M, Marousis CG, Nelson DR, Lauer G, González-Peralta R, Davis GL, et al. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol 1997; 26: 471-8. [ Links ]
8. Napoli J, Bishop GA, McGuiness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of a Th1-associated cytokines. Hepatology 1996; 24: 759-65. [ Links ]
9. Dumoulin FL, Bach A, Leifeld L, El Bakri M, Fisher H. pylori, Sauerbruch T, et al. Semiquantitative analysis of intrahepatic cytokine mRNAs in chronic hepatitis C. J Infect Dis 1997; 175: 681-5. [ Links ]
10. Cribier B, Schmitt C, Rey D, Lang JM, Kirn A, Stoll-Keller F. Production of cytokines in patients infected by hepatitis C virus. J Med Virol 1998; 55: 89-91. [ Links ]
11. Malaguarnera Z, Di Fazio I, Laurino A, Pistone G, Restuccia S, Trovato BA. Decrease of interferon gamma serum levels in patients with chronic hepatitis C. Biomed Pharmacother 1997; 51: 391-6. [ Links ]
12. Bozkaya H, Bozdayi AM, Aslan N, Turkay C, Sarioglu M, Centinkaya H, et al. Circulating IL-2 and IL-10 in chronic active hepatitis C with respect to the response to IFN treatment. Infection 2000; 28: 309-13. [ Links ]
13. Cacciarelli TV, Martínez OM, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: Pre- and posttreament with interferon alfa. Hepatology 2002; 24: 6-9. [ Links ]
14. Vrolijk JM, Kwekkeboom J, Janssen HL, Hansen BE, Zondervan PE, Osterhaus AD, et al. Pretreatment intrahepatic CD8+ cell count correlates with virological response to antiviral therapy in chronic hepatitis C infection. J Infect Dis 2003; 188: 1528-32. [ Links ]
15. Masaki N, Fukushima S, Hayashi S. Lower Th-1/Th-2 ratio before interferon therapy may favor long-term virological responses in patients with chronic hepatitis C. Dig Dis Sci 2002; 47: 2163-9. [ Links ]
16. Marinho RT, Pinto RM, Santos ML, de Moura MC. Lymphocyte T helper-specific reactivity in sustained responders to interferon and ribavirin with negativization (seroreversion) of anti-hepatitis C virus. Liver Int 2004; 24: 413-8. [ Links ]
17. Francavilla V, Accapezzato D, De Salvo M, Rawson P, Cosimi O, Lipp M, et al. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur J Immunol 2004; 34: 427-37. [ Links ]
18. Shinohara M, Ishii K, Takanura N. Long term changes of peripheral blood CD4-positive T cell subsets (Th1,Th2) in chronic hepatitis C patients with a sustained response or no response to IFN. Hepatol Res 2003; 27: 260-5. [ Links ]
19. Bergamini A, Cepparulo M, Bolacchi F, Araco A, Tisone G, Ombres D, et al. Ribavirin increases mitogen and antigen-induced expression of CD40L on CD4+ T cells in vivo. Clin Exp Immunol 2002; 130: 293-9. [ Links ]
20. Lo Iacono O, García-Monzón C, Almasio P, García-Buey L, Craxi A, Moreno-Otero R. Soluble adhesion molecules correlate with liver inflammation and fibrosis in chronic hepatitis C treated with interferon-alpha. Aliment Pharmacol Ther 1998; 12: 1091-9. [ Links ]
21. García-Buey L, García-Monzon C, Moreno-Otero R. The mediators of inflammation and chronic hepatitis. Gastroenterol Hepatol 1995; 18: 42-54. [ Links ]
22. Neuman MG, Benhamou JP, Malkiewicz IM, Akremi R, Shear NH, Asselah T, et al. Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem 2001; 34: 173-82. [ Links ]
23. Esquivel F, Albillos A, Carrión F, Prieto A, Reyes E, Martínez-Martín B, et al. Relationship between response to interferon-alpha and function of peripheral blood mononuclear cells in chronic hepatitis C patients. Dig Dis Sci 2002; 47: 2154-62. [ Links ]
24. Adinolfi LE, Andreana A, Utili R, Zampino R, Ragone E, Ruggiero G. HCV RNA levels in serum, liver and peripheral blood mononuclear cells of chronic hepatitis C patients and their relationship to liver injury. Am J Gastroenterol 1998; 93: 2162-6. [ Links ]
25. Ladero Quesada JM. Chemokines in liver disease. A new field of study of genetic polymorphism? Rev Esp Enferm Dig 2003; 95: 603-6. [ Links ]











 texto en
texto en