Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.4 Madrid abr. 2009
Comparison of two vaccination strategies against hepatitis A and B in patients with chronic hepatitis C
Comparación de dos estrategias de vacunación frente a la hepatitis A y B en individuos con hepatitis crónica C
M. P. Díez Redondo, A. Almaraz1 M. Jiménez Rodríguez-Vila2, A. Santamaría, J. de Castro3, J. C. Torrego4 and A. Caro-Patón
Service of Digestive Diseases. Hospital Universitario Río Hortega.
1Department of Microbiology and Pre-emptive Medicine. School of Medicine.
2Primary Health Care. Health Center "Gamazo". Services of 3Clinical Analysis and 4Oncology. Hospital Universitario Río Hortega. Valladolid, Spain
ABSTRACT
Objective: although the vaccination against hepatitis A (VAH) and hepatitis B (VBH) is recommended in patients with HCV, the most cost-effective strategy has not been established. Our objective was to compare the cost-effectiveness of universal strategy (vaccination all patients) with selective strategy (vaccination only patients against virus they lack immunity to) in patients with HCV.
Patients and methods: we compared the direct medical costs of the two vaccination strategies against both viruses in 313 patients with HC. Serological markers for HAV (anti-HAV) and HBV (HbsAg, anti HBs, anti HBc) were determined in the 313 patients and the costs of the vaccines and the blood tests necessary to determinate the immunity state in our care system were considered.
Results: the prevalence of anti-HAV was 81,2% and of anti-HBc was 24,6%. The prevalence of anti-HAV increases with age. HAV vaccination with universal strategy has a cost of 19.806,64 € and with selective one of 9.899,62 €. HBV vaccination with universal strategy rose to 18.780 € and to 20.385,57 € with selective one (employing anti-HBc). Costs were analysed in different groups of age and several hepatitis HBV risk factors.
Conclusions: the selective vaccination strategy against HAV was most cost-effective in our patients with HCV. However, when the prevalence of the anti-HAV decreased to less than 20% universal strategy will be the best option. Difference of cost-effective between the two vaccination strategies against HBV was small, on behalf of universal one, so in groups with higher anti-HBc prevalence, like parenteral drugs users and tattoos, the selective strategy could be the best option.
Key words: HAV vaccine. HBV vaccine. Chronic hepatitis C. Cost-effectiveness.
RESUMEN
Objetivo: aunque se recomienda vacunar frente al virus de la hepatitis A (VHA) y al virus de la hepatitis B (VHB) a los pacientes con infección crónica por el virus de la hepatitis C (VHC), la estrategia de vacunación más costo-efectiva aún no está establecida.
Nuestro objetivo fue comparar la vacunación universal (de todos los individuos) con la selectiva (únicamente de los individuos no inmunizados) frente al VHA y al VHB de los pacientes con infección crónica por VHC en nuestro medio.
Pacientes y métodos: comparamos los costes directos de las dos estrategias de vacunación frente a ambos virus en 313 individuos con infección crónica por VHC. Determinamos los marcadores serológicos del VHA (anti-VHA) y del VHB (HBsAg, anti-HBs y anti-HBc) y tuvimos en cuenta los costes de vacunas y reactivos en nuestro ámbito.
Resultados: la prevalencia de anti-VHA fue del 81,2% y la de anti-HBc del 24,6%. La prevalencia de anti-VHA aumentaba progresivamente con la edad. La inmunización frente al VHA suponía 19.806,64 € con la estrategia universal y 9.899,62 € con la selectiva. La vacunación frente al VHB ascendía a 18.780 € con la inmunización universal y a 20.385,57 € con la selectiva (mediante el anti-HBc). Se analizaron los costes considerando distintos grupos etarios y diversos factores de riesgo.
Conclusiones: en nuestros individuos con infección crónica por VHC la vacunación selectiva frente al VHA es la más costoefectiva. Pero cuando el porcentaje de inmunización frente al VHA desciende por debajo del 20% la mejor opción es la universal. La diferencia en la costoefectividad de ambas estrategias de vacunación frente al VHB es pequeña, a favor de la universal, por lo que en subgrupos con elevada prevalencia de anti-HBc, como adictos a drogas y tatuados, la selectiva podría ser la mejor alternativa.
Palabras clave: Vacuna anti-VHA. Vacuna anti-VHB. Hepatitis crónica por VHC. Costo-efectividad.
Introduction
We currently recommend vaccination against the hepatitis A (HAV) and hepatitis B (HBV) viruses as a part of preventive measures for patients with chronic liver disease (1,2). This is due to the knowledge that the presence of an underlying liver disease, together with age, is one of the most important factors for increased morbidity and mortality after infection with these viruses.
Hepatitis by HAV is usually a self-limited condition with a fatality rate of 0.01 to 0.5% in adults. However, when it occurs in individuals with chronic HBV infection, mortality can be up to 50 times higher than expected in healthy individuals (3). In relation to chronic liver disease by HCV, a controversial study was published (4) reflecting the development of liver failure in 41.2% of patients with HCV-related chronic hepatitis who had acute infection with HAV, which led to death in 6 cases. Similarly, other studies have confirmed the worse course of acute hepatitis A when it occurs in individuals with chronic liver disease either secondary to alcohol or cryptogenetic in nature (5).
The consequences of acute HBV superinfection in patients with chronic liver disease by HCV are uncertain. Although some individuals experience a rapid progression to fulminant hepatic failure, others may present a resolution of both infections as reflected in a study (6); however, this has not been confirmed. Regarding chronic infection with HBV and HCV, studies have shown that these patients have more severe liver damage and more complications, including an increased frequency of hepatocellular carcinoma, when compared to non-coinfected patients (7,8).
Although in recent years the incidence of HAV and HBV infection has decreased in most industrialized countries, due to improved socio-economic conditions and various immunization programs, these conditions remain a very important public health concern, and an increase in hepatitis cases by these viruses has been reported in young adults, including some outbreaks (9-11).
At present we have vaccines against HAV and HBV that are safe, effective and well tolerated by healthy subjects and patients with chronic liver disease; however, in the latter effectiveness diminishes with disease progression (12,13), and so vaccines are recommended in the early stages of liver disease.
Due to the high cost of vaccination against HAV and HBV in patients with chronic liver disease by HCV the various health systems need to design the most cost-effective vaccination strategy and to evaluate the usefulness of identifying permanent immunity markers against these viruses as a precursor to vaccination of only non-immunized (selective strategy) subjects, as opposed to the vaccination of all subjects with chronic liver disease (universal strategy). In addition, to find the best strategy we need to know the prevalence of immunization against these viruses in patients with chronic HCV infection, which may differ from that of the general population due to certain characteristics in some of them, and also the costs of serum marker measurements and vaccines.
The objectives of our study have been to know the prevalence of immunization against HAV and HBV in individuals with chronic HCV liver disease, and to compare the costs of vaccination with the universal versus the selective strategy for each of these viruses in these patients.
Patients and methods
Between January 2003 and May 2004 we studied 313 adult patients with chronic HCV infection followed at the outpatient clinic, Gastroenterology Hospital Río Hortega, Valladolid.
We analyzed age, sex, alcohol consumption, presence of tattoos, injected drug users (IDUs), blood transfusions and surgery before 1990, and previous vaccination against HAV or HBV.
All of them had serum markers for HCV (anti-HCV) measured using microparticle enzyme immunoassay with the HCV 3.0 AXSYM autoanalyzer (Abbott Laboratories), for HAV (anti-HAV Ig G type) using an enzyme immunoassay with the autoanalyzer AXSYM Habab 2.0 (Abbott Laboratories), and for HBV (HBs Ag, anti-HBs, anti-HBc) using an immunoassay with reagents Elecsys electro-chemoluminiscence HBsAg, Elecsys Anti-HBs and anti-HBc, respectively, with the automatic analyzer Roche Elecsys 1010/2010.
For statistical analysis the data were processed using the Statistical Package for Social Sciences, version 12.0, considering statistical significance for an error α < 5%. We calculated the prevalence of anti-HAV and anti-HBc by age groups in patients with chronic HCV infection. Taking as reference value the younger group (≤ 40 years), we calculated the relative prevalence in the other age groups and their corresponding 95% confidence intervals.
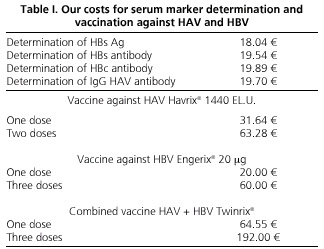
To make the cost-effective study of the two strategies of vaccination against hepatitis viruses A and B, the costs of the vaccines and reagents necessary for carrying out the serological screening in our area were considered, as well as the costs of the health staff responsible for drawing blood, serum findings, and vaccine administration (Table I). To minimize costs, serum tests in the selective strategy and the indication of vaccination in both were included in the routine medical program for the review of patients with chronic HCV infection, and the number of medical consultations for both strategies was the same. The study was approved by the Ethics Committee of our institution, and all patients consented to be part of it, and allowed the use of data collected in their medical records, and serum testing for hepatitis A and B where missing. It also met the current legislation on personal data protection.
Results
Of the 313 patients studied 185 were males and 128 females, with an average age of 50.7 years (range 24-82 years). A history of alcohol consumption, presence of tattoos, IDU status, and blood transfusion and surgery prior to 1990 were present in 24.9, 11.2, 21.1, 36.7, and 51.1% of cases, respectively. No patient had been vaccinated against HAV and 9 had received an anti-HBV vaccine.
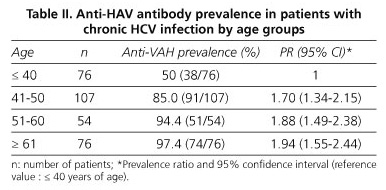
The prevalence of anti-HAV found in our patients with chronic HCV infection was 81.2%. Table II identifies that the prevalence of anti-HAV increases linearly with age, from 50% in those younger than 40 years to 97.45 in those in the 61 and older range. Taking as reference value the younger age group, the excess risk, measured by the relative prevalence, is 70, 88, and 94% in the three age groups, this being in all cases statistically significant.
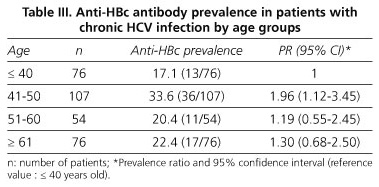
As for HBV markers, HBsAg was present in 1.3%, anti-HBs in 20.1%, and anti-HBc in 24.6% of cases. Regarding the relationship of anti-HBc with age, we noted that, unlike the previous situation with the prevalence of anti-HAV, the prevalence of anti-HBc has no linear trend in patients with chronic HCV infection. It is highest in the group of 41-50 years with a value of 33.6% and a PR of 1.96 (95% CI: 1.12-3.35) in relation to the group with ≤ 40 years. In other groups, prevalence ratios showed a clear trend that was not statistically significant (Table III).
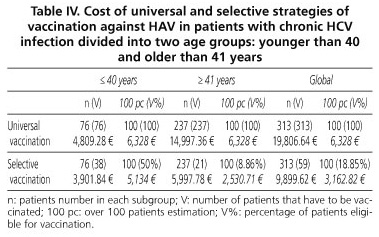
To assess the costs of vaccination against HAV for patients with chronic HCV infection, we calculated the differences between the use of a universal strategy, involving the vaccination of 313 patients with two doses of vaccine Havrix® 1440 (SmithKline Beecham Biologicals), which sums 19,806.64 €, with the implementation of a targeted strategy, whose overall cost of 9899.62 € would be the result of adding the costs of anti-HAV IgG measurement in 313 individuals (6,166.1 €) over the vaccination of 59 anti-HAV-negative individuals (3,733.52 €). Table IV lists the results of both strategies by age groups.
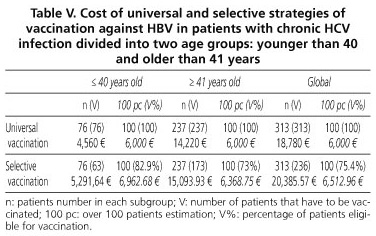
With regard to immunization against HBV in these patients, the universal vaccination of 313 patients with three doses of Engerix® 20 vaccine (GlaxoSmithKline) would cost 18,780 €, while the targeted strategy, using anti-HBc as a marker after immunization against HBV, would amount to 20,385.57 €, the result of aggregating the costs of measuring this marker in 313 patients (6,225.57 €) over the vaccination of 236 anti-HBc-negative patients (14,160 €). This is reflected by age groups in table V.
If the serologic screening is done by anti-HBc and anti-HBs, the selective strategy would cost 24,062.59 €, while 28,770.11 € is estimated for the combination of these two serum markers with HBsAg.
The use of the combined vaccine Twinrix® (GlaxoSmithKline), following a universal strategy with three doses, would cost 60,096 € for the 313 patients. This cost would drop to 33,721.19 € using the combined vaccine for the immunization of 50 selective patients identified as anti-HAV- and anti-HBc-negative, and the corresponding monovalent vaccine in individuals with a single negative serologic marker.
Discussion
While several studies (3,4) have shown an increased morbidity and mortality of HAV and HBV infections if they occur in individuals with chronic liver disease, and recommendations have been made thereon by various international health agencies (1,2), vaccines against these viruses in patients with chronic HCV infection are underused (14). One of the major obstacles that stand in the way of proper vaccination for these patients is the high cost that this preventive measure implies (15), making it vital to develop vaccination strategies that are more cost-effective.
In our series of patients with chronic HCV infection we found a rate of immunization against HAV of 81.2%, similar to that found by other authors (16). Because anti-HAV prevalence is lower among younger individuals, 50% of our patients with chronic HCV infection under 41 years were susceptible to acute infection with HAV, as described in an epidemiological study done by our group in this same population (17).
With regard to HBV, 24.6% of our patients with chronic HCV infection were anti-HBc-positive. This serologic marker shows no association with age and, although it is higher in those older than 41 years, the rise is not age-dependent.
Combining these percentages with the costs of immunization, serologic screening, and vaccination, we conclude that the strategy of vaccination against HAV that is most cost-effective for our patients with chronic HCV is the selective one, since it represents saving 9,907.02 € versus universal vaccination.
Furthermore, by comparing the two vaccination strategies in subjects younger than 40 years versus those older than 41 years (Table IV), we found that the increasing prevalence of anti-HAV according to age parallels the savings obtained by targeted vaccination. Therefore, in both groups targeted vaccination is most cost-effective; among those under 40 years the difference between costs for both strategies is already very low and, if we include a greater representation of younger age groups, there would be a point where universal vaccination would be most cost-effective. This occurs when the prevalence of anti-HAV was 31% lower.
Despite significant limitations due to the low number of patients younger than 30 years in our series, we observed that, whereas in the subgroup of 30 to 35 years the prevalence of anti-HAV was 47%, in those below 30 years it was less than 20%, namely 14%. Therefore, in our context, targeted vaccination against HAV is a cost-effective strategy for patients with chronic liver disease by HCV, probably except for those under 30 years, in whom universal vaccination might be more advisable.
Other previous studies have provided results similar to ours. A Spanish study (18) concluded that vaccination should be used selectively in those groups where the prevalence of anti-HAV exceeded 27%, and recommended universal vaccination for subjects under 24 years of age in one group at risk. As this study was published in 1995, the age gap between this and the findings in our work could be partly due to the gradual decline in natural immunization against HAV that is occurring in young individuals in developed countries.
More recently, a French study (19) showed that targeted vaccination of patients with chronic liver disease by HCV is more economical than universal vaccination, especially for those under 40 years.
Other American authors conclude, regarding the costs and prevalence of anti-HAV in their environment, that universal vaccination is recommended when the prevalence of anti-HAV is less than 12% (20), 22% (21), or 50% (22). Siddiqui et al. (21) conclude that targeted vaccination is particularly indicated in those older than 40 years and in Afroamericans, due to the high prevalence of anti-HAV in these subjects, whereas Duncan et al. (20) set the cut-off age at 30 years.
In two other studies (23,24), targeted vaccination against HAV for patients with chronic HCV infection has been cost-effective to reduce HAV-related morbidity and mortality.
Regarding vaccination against HBV in patients with chronic liver disease by HCV in our context, universal and selective strategies, using the anti-HBc, are very similar in terms of cost-effectiveness (18,780 € vs. 20,385.57 €) (Table V). However, with our costs, targeted vaccination would be most cost-effective from a percentage of positivity for anti-HBc of 32%. Using a combination of various serological markers of HBV raises the cost of selective vaccination well above universal vaccination.
Our results are consistent with those described previously by other authors. Thus, while the screening prior to vaccination against HBV is not cost-effective in the general population (25), it may be in patients with chronic liver disease by HCV (5,26) with the highest prevalence of immunization against HBV. In this respect two recent papers argue that targeted vaccination is recommended when the rate of immunization against HBV is greater than 10% (21) or 30% (27), respectively.
Furthermore, selective vaccination would be particularly profitable in the subgroups of patients with chronic HCV infection with a higher rate of immunization against HBV, including IDUs, male homosexuals, Asian-Americans, or immigrants from endemic areas (21,26,28). In our study we found a percentage of positive anti-HBc of 48.5% in IDUs (n = 66) and of 18.22% in non-IDUs (n = 247), whereas in carriers of tattoos (n = 35) this percentage was 45.7 versus 21.94% for non-tattooed individuals (n = 278). The antecedent of being an IDU and of having tattoos was significantly associated with the presence of anti-HBc in patients with chronic HCV infection (p = 0.0000 p = 0.0003, respectively), so that targeted vaccination may be most advisable.
There are different opinions about which is the best serum marker for prescreening in targeted immunization against HBV. The single measurement of anti-HBs may be insufficient because up to 5% of individuals may have isolated anti-HBc as only marker for HBV infection (29), which in most subjects means permanent immunity after a past infection but not anti-HBs positivity. Therefore some authors recommend the use of anti-HBc as a marker for immunization against HBV (21,28). However, their use alone does not distinguish between chronic hepatitis B virus infection or exposure to this virus after immunization, and there are false positive results, so other authors recommend HBsAg and anti-HBs (30), while others employ a combination of HBsAg, anti-HBc (Ig G), and anti-HBs (31). Our view is that it may suffice with anti-HBc screening since its absence identifies patients most likely to suffer acute HBV infection.
The use of a combined vaccine against HAV and HBV, although initially with higher costs, could be cost-effective by reducing the number of punctures and visits to clinics, and improving the compliance of patients is a determinant factor to increase cost-effectiveness (32).
It should also be noted that public health centers where the costs of health staff can be included within the activity of each center, the selective strategy achieved a greater cost, although a recent study (33) looking for the best vaccination strategy against HAV and HBV infection in patients with chronic HCV concluded that, although selective vaccination is most cost-effective, universal vaccination may achieve greater efficiency to immunize more patients with an increase in cost per patient that is small and manageable, taking into account the morbidity and mortality prevented.
In any case it seems clear that we need further studies to assess, among other things, the indirect costs of each strategy in order to allow for more precise recommendations.
![]() Correspondence:
Correspondence:
Mª Pilar Díez Redondo.
Servicio de Aparato Digestivo.
Hospital Universitario Río Hortega.
C/ Dulzaina, s/n.
47012 Valladolid, Spain.
e-mail: diezmp@hotmail.com
Received: 17-11-08.
Accepted: 12-02-09.
References
1. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006; 55(RR07): 1-23. [ Links ]
2. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002-June 10-12, 2002. Hepatology 2002; 36(5 Supl. 1): S3-20. [ Links ]
3. Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol 1995; 90: 201-5. [ Links ]
4. Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 1998; 338: 286-90. [ Links ]
5. Akriviadis EA, Redeker AG. Fulminant hepatitis A in intravenous drug users with chronic liver disease. Ann Intern Med 1989; 110: 838-9. [ Links ]
6. Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol 2000; 95: 2978-80. [ Links ]
7. Alberti A, Pontisso P, Chemello L, Fattovich G, Benvegnu L, Belussi F, et al. The interaction between hepatitis B virus and hepatitis C virus in acute and chronic liver disease. J Hepatol 1995; 22(1 Supl.): 38-41. [ Links ]
8. Benvegnu L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrosis. A prospective study. Cancer 1994; 74: 2442-8. [ Links ]
9. Wiwanitkit W. Hepatitis A oubreak in Thailand during the past 25 year period, a summary. Rev Esp Enferm Dig 2008; 100: 115-6. [ Links ]
10. Mele A, Rastelli MG, Gill ON, Di Bisceglie D, Rosmini F, Pardelli G, et al. Recurrent epidemic hepatitis A associated with consumption of raw shellfish, probably controlled through public health measures. Am J Epidemiol 1989; 130: 540-6. [ Links ]
11. Wang HY, Hun SL, Lin NY, Hong YL, Cao SZ, Win LF. Risk factors analysis of an epidemic of hepatitis A in a factory in Shanghai. Int J Epidemiol 1990; 19: 435-8. [ Links ]
12. Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998; 27: 881-6. [ Links ]
13. Idilman R, De Maria N, Colantoni A, Nadir A, Van Thiel DH. The effect of high dose and short interval HBV vaccination in individuals with chronic hepatitis C. Am J Gastroenterol 2002; 97: 435-9. [ Links ]
14. Oldfield EC3 rd, Keeffe EB. The A' and B' of vaccine-preventable hepatitis: improving prevention in high-risk adults. Rev Gastroenterol Disord 2007; 7(1): 1-21. [ Links ]
15. Bockhold K, Riely CA, Jeffreys Ch. Overcoming obstacles to immunization in patients with chronic liver disease. Am J Med 2005; 118: 40S-45S. [ Links ]
16. López Serrano A, Antón Conejero MD, Alcaraz Soriano MJ, García del Castillo G, Gómez Pajares F, Moreno-Osset E. Prevalence of hepatitis A virus antibodies in patients with chronic liver diseases. Rev Esp Enferm Dig 2000; 92: 508-517. [ Links ]
17. Díez Redondo MP, Almaraz A, Jiménez Rodríguez-Vila M, Santamaría A, De Castro J, Torrego JC, et al. Inmunidad frente al virus de la hepatitis A en los pacientes con infección crónica por el virus de la hepatitis C. Med Clin (Barc) 2008; 131: 526-9. [ Links ]
18. Navas E, Bayas JM, Bruguera M, Vidal J, Gali N, Taberner JL. Eficiencia de la detección prevacunal de anti-VHA en los programas de vacunación antihepatitis A. Med Clin (Barc) 1995; 105: 168-71. [ Links ]
19. Rufat P, Dumouchel P, Cadranel JF. Séroprévalence de l'infection par le virus de l'hépatite A et évaluation du coût direct de diferentes stratégies vaccinales contre le virus de l'hépatite A chez les malades atteints d'hépatite chronique C en France. Gastroenterol Clin Biol 2002; 26: 256-60. [ Links ]
20. Duncan M, Hirota WK, Tsuchida A. Prescreening versus empirical immunization for hepatitis A in patients with chronic liver disease: a prospective cost analysis. Am J Gastroenterol 2002; 97: 1792-5. [ Links ]
21. Siddiqui F, Mutchnick M, Kinzie J, Peleman R, Naylor P, Ehrinpreis M. Prevalence of hepatitis A virus and hepatitis B virus immunity in patients with polymerase chain reaction-confirmed hepatitis C: implications for vaccination strategy. Am J Gastroenterol 2001; 96: 858-63. [ Links ]
22. Saab S, Martin P, Yee H. A simple cost-decision analysis model comparing two strategies for hepatitis A vaccination. AM J Med 2000; 109: 241-3. [ Links ]
23. Jacobs RJ, Koff RS, Meyerfoff AS. The cost-effectiveness of vaccinating chronic hepatitis C patients against hepatitis A. Am J Gastroenterol 2002; 97: 427-34. [ Links ]
24. Arguedas MR, Heudebert GR, Fallon MB, Stinnett AA. The cost-effectiveness of hepatitis A vaccination in patients with chronic hepatitis C viral infection in the United States. Am J Gastroenterol 2002; 97: 721-8. [ Links ]
25. Blostein J, Clark PA. Cost-effectiveness of preimmunization hepatitis B screening in high-risk adolescents. Public Health Rep 2001; 116: 156-68. [ Links ]
26. Keefe EB. Vaccination against hepatitis A and B in chronic liver disease. Viral Hepatitis Rev 1999; 5: 77-88. [ Links ]
27. Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med 1997; 33: 196-204. [ Links ]
28. Reiss G, Keeffe EB. Hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther 2004; 19: 715-27. [ Links ]
29. Colomina-Rodríguez J, González-García D, Burgos-Teruel A, Fernández-Lorenz N, Guerrero-Espejo A. Significado de la reactividad aislada anti-HBc como único marcador de infección de la hepatitis B. Enferm Infecc Microbiol Clin 2005; 23: 80-5. [ Links ]
30. National Task Force for Hepatitis B: Focus on Asian and Pacific Islander Americans. Available at: http://www.hepbinitiative.org/taskforce/index.html. [ Links ]
31. Lau D, Hewlett A. Screening for hepatitis A and B antibodies in patients with chronic liver disease. Am J Med 2005; 118: 28S-33S. [ Links ]
32. Saab S, Martin P, Yee H. A simple cost-decision analysis model comparing two strategies for hepatitis A vaccination. AM J Med 2000; 109: 241-3. [ Links ]
33. Jakiche R, Borrego ME, Raisch DW, Gupchup GU, Pai MA, Jakiche A. The cost-effectiveness of two strategies for vaccinating US veterans with hepatitis C virus infection against hepatitis A and hepatitis B viruses. Am J Med Sci 2007; 333(1): 26-34. [ Links ]











 texto en
texto en