Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.102 no.11 Madrid nov. 2010
LETTERS TO THE EDITOR
Acute hepatitis caused by minocycline
Hepatitis aguda por minociclina
Palabras clave: Hepatitis aguda. Histología. Minociclina.
Key words: Acute hepatitis. Histology. Minocycline
Dear Editor,
Minocycline, a derivative of tetracycline, is an antibiotic commonly employed in the treatment of acne vulgaris for both its effectiveness and the long half-life (11-13 hours), which allows for a once to twice a day dosing schedule (1), and it accounts for 40% of the total tetracycline use (2). We describe a case of acute hepatitis caused by this antibiotic.
Case report
A 80 years old lady came to our attention in February 2007 due to dysphagia and increase of liver enzymes (AST 194 U/L, ALT 308 U/L). She had been treated with minocycline, 100 mg/day, from February 2005 to March 2006, for perioral dermatitis.
Upper endoscopy revealed a rabbit-bone impacted in the proximal third of the esophagus, removed with grasping forceps. Serological markers for hepatitis were negative; mild anemia (Hb 12.8 g/dl) and leukopenia (3.300/mmc) with a modest increase of gammaglobulines (20.4%) were noted. Anti-nuclear antibodies were present (1:160, homogenous pattern) while anti-mitochondrial, anti-liver-kidney and anti-endomysial antibodies were negative. No Ig deficit was present.
The patient was discharged with a follow-up program. After one month, liver enzymes increased (AST 652 U/L, ALT 721 U/L, GGT 107 U/L), as well as anti-nuclear antibodies (1:320), and the liver function was mildly impaired (prothrombin time 75% with INR of 1.22, bilirubin level 2.2 mg/dl with direct bilirubin 0.7 mg/dl, cholinesterases 4939 U/L).
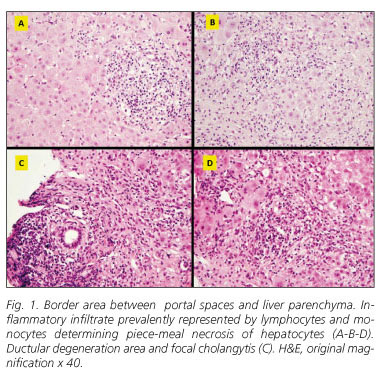
Liver biopsy revealed intact lobular architecture with diffuse dilation of portal spaces, inflammatory infiltrate prevalently represented by lymphocytes and monocytes, and septs development. Ductular degeneration area, focal cholangytis, severe piece-meal necrosis, several areas of lobular necrosis (zone 3) and perivenular necrosis with portal-central bridges were found (Fig. 1). Prednisone, 50 mg/day, was started with a low tapering schedule and, after one year, the patient showed a complete normalization of liver enzymes. In June 2008, steroid therapy was stopped and the patient is presently enjoying good health.
Discussion
Drug-induced hepatitis is an uncommon entity in clinical hepatology but does represent a significant proportion of acute self-limited liver disease (3). The exact mechanism is not clearly understood, but many antibiotics, or their active metabolites, can bind to cellular macromolecules forming neo-antigens that appear extraneous to the immune system; the immune responses against such self components can trigger auto reactions to their unmodified counterpart and/or other auto antigens through epitope spreading (4).
Hepatotoxicity of tetracyclines was first described soon after the introduction in 1950, with minocycline being the most widely incriminated molecule. Since then, other cases have been described (5,6); it may be estimated that the overall literature report of minocycline hepatitis includes less than 200 cases.
Three types of minocycline-induced hepatitis have been described: typical toxin-mediated hepatic injury; fulminant hepatic failure as part of an allergic, idiosyncratic reaction chronic active hepatitis (CAH) detected by biopsy with positive ANA antibodies.
Given the possibility of liver injury related to minocycline use, it is important for dermatologists and pediatricians to be aware of this important side effects of minocycline therapy. Currently, there is no way to predict which patients are at risk to develop autoimmune hepatitis after exposure to the drug. A careful drug history should always be considered in any patient with chronic hepatitis because minocycline is often wrongly considered a cosmetic medicine and, in this particular type of patients, may determine an irreversible liver failure. An early recognition and discontinuation of the drug is important to aid recovery and to avoid the use of steroid and/or immunosuppressive agents.
Elderly patients may have an indolent progressive disease that is clinically asymptomatic. An acute, even fulminant, presentation may reflect a "de novo" disease or an exacerbation of a pre-existent chronic process. Centrilobular (zone 3) necrosis reflects an early histological stage that may evolve later in classical interface hepatitis. Prednisone (also in combination with azathioprine) is the safest effective initial treatment and all elderly patients with severe disease should be treated vigorously even though immunosuppressive medications, especially when used in combination are associated with an increased risk of opportunistic infections.
In conclusion, physicians should be aware of liver injury related to minocycline, since this side effect, although generally benign, may sometimes evolve in serious, even fatal hepatic insufficiency. Thus, a prompt recognition of potential liver injury related to the use of this drug is mandatory, in order to start as soon as possible an adequate therapeutic regimen and avoid more serious complications.
G. Casella1, V. Villanacci2, C. Di Bella2, E. Drera3, V. Baldini1, G. Bassotti4
1Division of Internal Medicine and 2Pathology. Desio General Hospital. 32nd Pathology Section. Spedali Civili di Brescia;
and 4Gastroenterology and Hepatology Section. Department of Clinical and Experimental Medicine. University of Perugia. Italy
References
1. Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin 2009; 27: 33-42 [ Links ]
2. Ford TJ, Dillon JF. Minocycline hepatitis. Eur J Gastroenterol Hepatol 2008; 20: 796-9. [ Links ]
3. Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center (corrected). J Clin Gastroenterol 2005, 39:64-67 Erratum in: J Clin Gastroenterol 2005; 39: 176. [ Links ]
4. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther 2007; 25: 1135-51. [ Links ]
5. Real Martínez Y, Medina S. Acute hepatitis and minocycline. Rev Esp Enferm Dig 2003; 95: 741-2. [ Links ]
6. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol 2009; 43: 787-90. [ Links ]