Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.6 Madrid jun. 2015
ORIGINAL PAPERS
Feasibility of endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) and biopsy (FNB) with a new slim linear echoendoscope
Julio Iglesias-García1, Jose Lariño-Noia1, Nicolau Vallejo-Senra1, Daniel de-la-Iglesia-García1, Ihab Abdulkader-Nallib2 and J. Enrique Domínguez-Muñoz1
1Department of Gastroenterology and Foundation for Research in Digestive Diseases (FIENAD). University Hospital of Santiago de Compostela. A Coruña, Spain.
2Department of Pathology. University Hospital of Santiago de Compostela. A Coruña, Spain
ABSTRACT
Background: Endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) and biopsy (FNB) is considered a very accurate and safe tool for sampling extra-intestinal tumors. Standard echoendosocopes for FNA/FNB are large with a sharpened tip that can be associated with complications. A new slim linear echoendoscope have been developed trying to overcome this limitation.
Aim: Of the present study was to evaluate the feasibility; safety and diagnostic yield of this newly developed slim echoendoscope for performing EUS-guided FNA/FNB.
Methods: A pilot observational study was performed. Consecutive patients submitted for a EUS-FNA/FNB were prospectively included in the study. Patients underwent EUS procedure using the new slim linear PENTAX-echoendoscope. Tissue acquisition was done with standard and histology needles. Feasibility and diagnostic yield were evaluated. A descriptive analysis was performed.
Results: 87 patients were included (mean age 66.7 years (range 24-90 years), 45 male. Mean size was of lesions sampled were 33.43 ± 20.8 mm. Esophagus intubation and access to the second portion of the duodenum (D2) were considered easy in all 87 cases (100%). Nineteen procedures (21.8%) were performed from the esophagus, 42 (48.3%) from the stomach, 22 (25.3%) cases from duodenal bulb, and 4 (4.6%) cases from D2. EUS-FNB was feasible in 85 cases (97.7%), failed in 2 pancreatic lesions accessed from D2. Diagnostic yield was 86.21% (95%CI 77.4-91.9) in the intention-to-treat analysis and 88.24% (95%CI 79.7-93.5) in per-protocol analysis. There were no complications related to the technique.
Conclusion: Performing a EUS-FNA/FNB with the newly designed slim scope is feasible and safe for cyto-histopathology diagnosis of intra-intestinal and extra-intestinal mass lesions.
Key words: Endoscopic ultrasound. Fine needle biopsy. Slim echoendoscope.
Introduction
Endoscopic ultrasound (EUS) is considered as a very accurate method for detecting intra-intestinal and extra-intestinal lesions and lymph nodes (1-4). EUS-guided fine needle aspiration (FNA) and biopsy (FNB) provide with a high diagnostic accuracy, ranging from 60% to 90% (5-10).
However, there are certain drawbacks related to the technique, mainly due to potential complications. Those complications can be associated either with the endoscopic procedure or with FNA/FNB (11-14). Standard linear echoendoscopes required for EUS-guided FNA/FNB use to be larger than standard scopes in terms of outer diameter. Linear scopes also present a sharpened tip corresponding to the ultrasound transducer. Oral intubation and access to the second portion of the duodenum can be difficult with these large and sharpened scopes, which may be associated with severe complications such as perforation of piriform sinus or duodenum (15,16). In addition, due to the lack of deformation of the tip of the scope, FNA/FNB from the duodenum can be difficult to perform.
In order to avoid difficulties and complications associated with the size of linear echoendoscopes, a new slim linear echoendoscope (PENTAX Ultrasound gastroscope EG3270UK (HOYA Corporation, Tokyo, Japan), with standard working channel allowing the use of EUS-guided FNA/FNB needles, has been developed. The aim of the present study was to evaluate the feasibility, and yield of this newly developed echoendoscope for performing EUS-guided FNA/FNB. As a secondary objective we also evaluated its diagnostic accuracy targets included extra-intestinal solid lesions and peri-intestinal lymph nodes.
Material and methods
Design
A prospective and consecutive pilot observational study on the feasibility, safety and diagnostic accuracy of the EUS-guided FNA/FNB using the new slim linear echoendoscope for the evaluation of extraintestinal lesions and peri-intestinal lymph nodes was designed.
Subjects
EUS-guided FNA/FNB, from any location, performed between February and May of 2013 at the EUS Unit of the Department of Gastroenterology of the University Hospital of Santiago de Compostela, Spain, with the new slim echoendoscope (EG3270UK (HOYA Corporation, Tokyo, Japan), were included in the study.
Methods
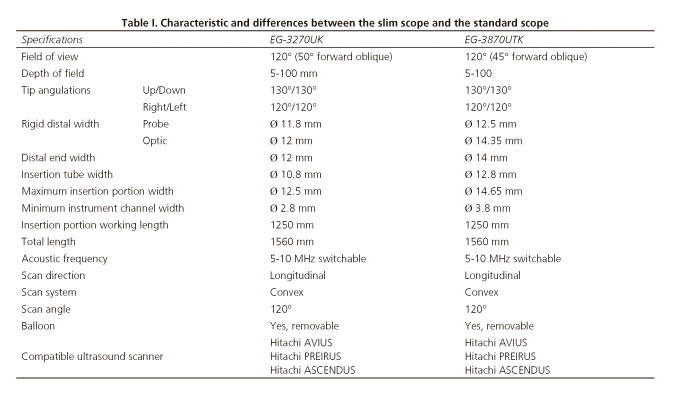
Patients underwent EUS procedure using a new linear PENTAX Ultrasound echoendoscope EG3270UK (HOYA Corporation, Tokyo, Japan) in combination with Hitachi Ultrasound scanner PREIRUS (Hitachi, Tokyo, Japan) (Fig. 1). Technical characteristics of the new scope and differences with the standard therapeutic scope are shown in table I.
Tissue acquisition was done with different EUS needles, both standard cytology and histology needles (Cook Endoscopy Inc, Limerick, Ireland), according to a standard protocol. Technical details of the standard tissue acquisition protocol were as follows: After the target lesion was endosonographically visualized and the region was scanned for vessels using color and pulsed Doppler, puncture was performed either from duodenum, stomach or esophagus according to lesion location. The needle to be used for each case was selected according to puncture site and lesion size. The needle was advanced into the target tissue under EUS guidance. Once the lesion was penetrated, the stylet was removed and suction was applied for 10 to 20 seconds using a 10 mL syringe while moving the needle to and fro within the lesion 3-4 times. Suction was released before removing the needle. Tissue samples were recovered into ThinPrep® (Hologic Corp, Bedford, MA) by flushing the needle with 5 cc of saline. Pathologists were not present in the endoscopy room and biopsy samples were recovered by the endoscopist and processed at the Pathology Department for histological analysis. Tissue samples were evaluated by the same and dedicated pathologist with particular interest and expertise in evaluating tissue materials obtained during EUS. Samples were embedded in paraffin. Tissue sections of 3-4 µ were stained with hematoxylin-eosin for morphological evaluation and different immunohistochemical analysis (cytokeratine, CD56, chromogranin and synaptophysin for endocrine tumors; CD20, CD3, bcl2, for lymphoma, or cytokeratin and TTF1 for non-small cell lung cancer) were performed as required. If pathologists were not able to obtain a core for histological evaluation, they processed the same material as cellblock for cytological evaluation.
Variables evaluated
Main variables evaluated were feasibility and safety of the procedure. Feasibility was defined as the possibility of performing the complete procedure, obtaining samples for cytohistological evaluation. With this aim, ease of esophagus intubation, ease of duodenal access, ultrasound quality image, scope flexibility, ease of positioning the scope for the FNB, ease of FNB needle insertion through the scope, visibility of the needle during the puncture, ease of FNB needle removal from the scope, and number of needle passes were evaluated. Safety was defined as the absence of complications, both during and after the procedure. Patients were monitored at the endoscopy unit for two hours after the procedure for the evaluation of complications. Further follow-up was performed by evaluating the electronic clinical record of the patients at days 3, 7 and 15 after the EUS-guided FNB. Electronic clinical record includes any clinical episode of every patient being attended at any hospital of the regional health system, and therefore is a highly reliable tool for checking for any clinical event both related and unrelated with the procedure. Secondary outcome was to determine the percentage of cases in which a correct final cyto/histological diagnosis, according to the gold standard, was obtained.
Reference diagnosis of malignant versus benign disease
A final diagnosis of malignancy or benignancy was made according to one of the following reference methods: a) Definite benign or malignant histological diagnosis based on surgical resection specimens from operated patients; b) Cytology or histology findings with definite proof of malignancy and compatible clinical follow-up in patients with unresectable tumors according to EUS and CT scan findings; and c) cytology or histology findings without proof of malignancy and a minimum clinical follow-up time of 12 months for a final diagnosis of benign disease.
Data analysis
Results are shown as percentage and 95% confidence interval (CI). Normally distributed variables are presented as mean with standard deviation and range. A descriptive analysis is performed. Overall accuracy was calculated.
Ethical issues
The study was approved by the local institutional review board and conducted in accordance with the Declaration of Helsinki and its amendments, and Good Clinical Practice guidelines. All patients provided written informed consent to the study. EUS and EUS-FNA for the evaluation of intraintestinal or extraintestinal mass lesions and/or peri-intestinal lymph nodes are routine procedures in clinical practice. The use of the new slim scope instead of the standard echoendoscope adds no additional risk or inconvenience to the patient. The study followed the STROBE recommendations for observational studies.
Results
A total of 87 patients were finally included in the study, mean age 66.7 years (range 24-90 years), 45 male. No single lesion in the study period was excluded. Mean size of lesions evaluated was 33.43±20.8 mm. Demographics of patients included in the study are shown in table II.
Esophagus intubation and access to duodenum through pylorus was considered easy in all 87 cases (100%). Ultrasound B-mode image was considered optimal for performing the EUS-FNA/FNB in all 87 cases, independently of the location of the lesion. Positioning of the scope at different sites for EUS-FNA/FNB was easy in all cases (100%).
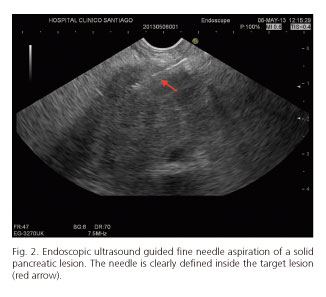
EUS-FNA/FNB was feasible in 85 cases (97.7%). Nineteen procedures (21.8%) were performed from the esophagus, 42 (48.3%) from the stomach, 22 (25.3%) cases from duodenal bulb, and 4 (4.6%) cases from second portion of the duodenum. Failures of procedure were in 2 pancreatic lesions located at uncinate process (accessed by second portion of the duodenum). Visibility of the needle during the EUS-FNB was considered excellent (Fig. 2). In all cases, both stylet and needle were easy to remove after the puncture. Mean number of needle passes was 1.4 (range 1-3). Distribution of needles used for EUS-FNA/FNB is also shown in table II.
EUS-FNA/FNB allowed obtaining a cyto-pathological sample in the 85 cases in which the procedure was completed. In terms of accuracy, diagnostic yield by an intention to treat analysis was 86.21% (95%CI 77.4-91.9) and per protocol, diagnostic yield reached was 88.24% (95%CI 79.7-93.5). Diagnosis obtained by EUS-FNA/FNB with the new slim scope, and final diagnoses according to gold standard are shown in table III.
There were no complications related to the technique. We had one perforation of the scope-working channel when targeting a lesion located at the second portion of the duodenum.
Discussion
This is the first study showing the feasibility and safety of EUS-FNA/FNB with a newly designed slim echoendoscope. All needle sizes and types available nowadays; both standard cytology ones and the new histology needles can be to be used through this new echoendoscope. The scope also facilitated a comfortable intubation of the patient and also an easy advancement up to the duodenum, especially when passing the pylorus. It allowed an optimal visualization of the region of interest with an optimal ultrasound image quality. Related to maneuverability characteristics, we have been able to puncture lesions from all upper gastrointestinal locations, from esophagus to deep portions of the duodenum. The overall diagnostic accuracy of the pathological assessment of the tissue samples obtained by FNA/FNB with this new slim echoendoscope was of 88.2% in per protocol analysis and 86.2% in the intention to treat evaluation.
With the development of this new echoendoscope, the first issue was to evaluate the feasibility of performing a EUS-FNA/FNB, because of the smaller working channel (2.8 mm in diameter), compared to the standard therapeutic scope (3.8 mm). We report for the first time on the feasibility of this new slim scope for performing EUS-FNA/FNB. In this context, we were able to perform all FNA/FNB procedures during the study period. A second important issue to be discussed is safety. Safety of EUS-FNA is well established, and complication rates range between 1% and 2.5% (11-13). However, there are certain critical points that need to be emphasized. Esophageal intubation is difficult in some cases. Cases of perforation of cervical esophagus at the time of intubation with a curvilinear echoendoscope have been described (0.06%), mainly related to the size of the scope (15). In our experience with the new slim scope, esophageal intubation was considered very easy and low traumatic in all cases. Another limitation of standard linear EUS is the advancement into the second portion of the duodenum, which is related to the stiffness of the tip of the standard echoendoscope. This is associated with complications such as perforation (14,16). Even more, the rigidity of the standard scope may preclude the possibility of bending the scope to access some lesions located in the duodenum and uncinate process of the pancreas. In our experience with the new slim scope, accessing the pylorus and deep portions of the duodenum was very simple in all cases. Performing the EUS-FNA/FNB from both duodenal bulb and second portion of duodenum was feasible and safe. In fact, we did not have any complication in all 87 cases included in the study.
EUS-guided tissue sampling has emerged as a valuable and essential technique for different indications. In fact, EUS-FNA has been proven to be a safe and useful method for tissue sampling of intramural and extramural gastrointestinal lesions (5,17). An adequate cytological specimen can be obtained in 82-91 % of cases, providing a diagnostic sensitivity for malignancy ranging from 64 to 96% (7-10,18-21). We have obtained similar data in our study, with an overall diagnostic yield of 88.2% in per protocol analysis. In previous studies, the recommended number of passes to obtain an optimal result is 5 to 7 for solid pancreatic lesions and 2 to 3 for lymph nodes. This is different in those cases where an on-site pathological evaluation is available (22). In our series, only 1.4 passes were necessary without on-site evaluation to obtain the accuracy reported. In this setting, the use of the new slim scope was associated with similar accuracy, and fewer needles passes, to those described in the literature with the standard scope.
There are different types of needles available for performing a EUS-FNA/FNB, both for obtaining cytological samples (18,23,24), or histological samples (6,25-27). An important point in our study is that we have been able to all kind of needles, including the bigger ones without concerns.
Another point to be commented is the use and definition of the criterion-standard reference method. Ideally, when the pathology results of the EUS-FNB are negative, histological confirmation from surgical specimens would be the criterion standard, which cannot be obtained for ethical reasons in patients in whom surgery is not indicated. In these specific cases, clinical follow-up for at least 12 months with repeated imaging procedures (EUS and CT) was used in our study. This method is a well-accepted reference standard (6,28,29).
In conclusion, performing EUS-guided biopsy with the new slim scope for histopathology diagnosis of intra-intestinal and extra-intestinal lesions is feasible and safe. It offers the possibility of performing a EUS-FNB with all needle sizes available, with an overall diagnostic accuracy for malignancy of over 86%. EUS-FNB with this new slim scope can be considered a valid option in clinical routine. These results deserve further confirmation in larger series and multicenter studies.
References
1. Iglesias Garcia J, Larino Noia J, Dominguez Munoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig 2009;101:631-8. [ Links ]
2. Iglesias-Garcia J, Lindkvist B, Larino-Noia J, et al. The role of EUS in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma. Rev Esp Enferm Dig 2012;104:315-21. [ Links ]
3. Shami VM, Waxman I. Technology insight: Current status of endoscopic ultrasonography. Nat Clin Pract Gastroenterol Hepatol 2005;2:38-45. [ Links ]
4. Dye CE, Waxman I. Endoscopic ultrasound. Gastroenterol Clin North Am 2002;31:863-79. [ Links ]
5. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011;43:897-912. [ Links ]
6. Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc 2011;73:1189-96. [ Links ]
7. Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, et al. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol 2007;13:289-93. [ Links ]
8. Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc 2012;75:319-31. [ Links ]
9. Savides TJ, Perricone A. Impact of EUS-guided FNA of enlarged mediastinal lymph nodes on subsequent thoracic surgery rates. Gastrointest Endosc 2004;60:340-6. [ Links ]
10. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc 2010;71:913-9. [ Links ]
11. Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy 2012;44:190-206. [ Links ]
12. Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622-9. [ Links ]
13. Adler DG, Jacobson BC, Davila RE, et al. ASGE guideline: Complications of EUS. Gastrointest Endosc 2005;61:8-12. [ Links ]
14. Mortensen MB, Fristrup C, Holm FS, et al. Prospective evaluation of patient tolerability, satisfaction with patient information, and complications in endoscopic ultrasonography. Endoscopy 2005;37:146-53. [ Links ]
15. Eloubeidi MA, Tamhane A, Lopes TL, Cervical esophageal perforations at the time of endoscopic ultrasound: A prospective evaluation of frequency, outcomes, and patient management. Am J Gastroenterol 2009;104:53-6. [ Links ]
16. Bournet B, Migueres I, Delacroix M, et al. Early morbidity of endoscopic ultrasound: 13 years' experience at a referral center. Endoscopy 2006;38:349-54. [ Links ]
17. Erickson RA. EUS-guided FNA. Gastrointest Endosc 2004;60:267-79. [ Links ]
18. Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc 2011;74:504-10. [ Links ]
19. Hollerbach S, Willert J, Topalidis T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: Histological and cytological assessment. Endoscopy 2003;35:743-9. [ Links ]
20. Catalano MF, Sial S, Chak A, et al. EUS-guided fine needle aspiration of idiopathic abdominal masses. Gastrointest Endosc 2002;55:854-8. [ Links ]
21. Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology 2003;125:1626-35. [ Links ]
22. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011;106:1705-10. [ Links ]
23. Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am 2012;22:155-67. [ Links ]
24. Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol 2013;48:752-7. [ Links ]
25. Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with a EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003;57:101-6. [ Links ]
26. Fernandez-Esparrach G, Sendino O, Sole M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy 2010;42:292-9. [ Links ]
27. Petrone MC, Poley JW, Bonzini M, et al. Interobserver agreement among pathologists regarding core tissue specimens obtained with a new endoscopic ultrasound histology needle; a prospective multicentre study in 50 cases. Histopathology 2013;62:602-8. [ Links ]
28. Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc 2009;70:1101-8. [ Links ]
29. Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: An accurate method for the differentiation of solid pancreatic masses. Gastroenterology 2010; 139:1172-80. [ Links ]
![]() Correspondence:
Correspondence:
Julio Iglesias-García.
Gastroenterology Department.
Foundation for Research in Digestive Diseases (FIENAD).
University Hospital of Santiago de Compostela.
c/ Choupana, s/n.
15706 Santiago de Compostela.
A Coruña, Spain
e-mail: julio.iglesias.garcia@sergas.es
Received: 14-12-2014
Accepted: 06-04-2015