Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 no.12 Madrid dic. 2015
ORIGINAL PAPERS
Incidence, clinical outcomes, and therapeutic approaches of capsule endoscopy-related adverse events in a large study population
Ignacio Fernández-Urien1, Cristina Carretero2, Begoña González3, Vicente Pons4, Ángel Caunedo5, Julio Valle6, Eduardo Redondo-Cerezo7, Antonio López-Higueras8, Mariano Valdés9, Pedro Menchen10, Pedro Fernández11, Miguel Muñoz-Navas2, Javier Jiménez1 and Juan Manuel Herrerías5
Department of Gastroenterology.
1 Complejo Hospital de Navarra. Pamplona, Spain.
2 Universidad de Navarra. Pamplona, Spain.
3 Hospital Clínic. Barcelona, Spain.
4 Hospital Universitario y Politécnico La Fe. Valencia, Spain.
5 Hospital Universitario Virgen Macarena. Sevilla, Spain.
6 Complejo Hospitalario de Toledo. Toledo, Spain.
7 Hospital Virgen de las Nieves. Granada, Spain.
8 Hospital General Universitario Morales Messeguer. Murcia, Spain.
9 Hospital Universitario Virgen de la Arrixaca. Murcia, Spain.
10 Hospital General Universitario Gregorio Marañon Hospital. Madrid, Spain.
11 Hospital Universitario Marqués de Valdecilla. Santander, Spain
ABSTRACT
Introduction: Capsule endoscopy (CE) has become a first-line tool for small bowel (SB) examination. However, adverse events (AEs), such as CE retention or aspiration, may occur. The aims of this study were to evaluate incidence, clinical outcomes and therapeutic approaches of CE-related AEs in the largest series published to date.
Methods: Data from 5428 procedures performed at 12 institutions between August 2001 and January 2012 were retrospectively analyzed. Baseline patient characteristics; procedure; type, localization and symptoms before/after AEs; previous patency tests performed; therapeutic management and patient's outcome were recorded.
Results: The overall incidence of CE-related AEs was 1.9%: 2.0% for SB, 0.9% for esophageal and 0.5% for colon CE. The incidence of capsule retention was significantly higher than capsule aspiration (1.87% vs. 0.003%; p < 0.05), in patients suffering from inflammatory bowel disease (IBD) than in obscure GI bleeding (OGIB) (3.3% vs. 1.5%; p < 0.05) and in patients with the combination of nausea/vomiting, abdominal pain and distension. The SB was the most frequent localization of retention (88.2%). The use of patency tests -except for Patency© capsule- before CE was not a good predictor for AEs. Most of the patients with AEs developed no or mild symptoms (97%) and were managed by non-surgical methods (64.4%).
Conclusions: CE-related AEs are uncommon and difficult to predict by imagiological examinations. SB retention, that is usually asymptomatic, is the most frequent AE. In absence of symptoms, non-surgical management of CE-related AEs is recommended.
Key words: Capsule endoscopy. Adverse events. Small bowel. Retention. Aspiration.
Introduction
Since its introduction in 2001 (1), capsule endoscopy (CE) has demonstrated to be an accurate, painless and safe procedure for patients (2-4). In fact, it is being currently considered as a first line diagnostic tool for small bowel examination. However, adverse events (AEs) during CE procedures such as capsule retention or aspiration can occur, ranging from 0% in healthy volunteers to 21% in those patients with suspected small bowel obstruction (2,5-8). Since most of CE-related AEs have been published as case reports or case-series (9-13), there is a lack of consensus on this topic and many questions remain open. A better knowledge of CE-related AEs could have a positive influence in patients' outcome. The aims of the present study were to evaluate the incidence, related factors, clinical course and therapeutic approach of CE-related AEs in the largest series published so far.
Patients and methods
Data from all CE procedures performed at 12 institutions all around Spain between August 2001 and January 2012 were retrospectively analyzed. In order to simplify and facilitate data compilation, a basic questionnaire containing standardized questions and answers specially designed to obtain the target information was developed. All participants were asked to search for the requested information in databases and/or patients' medical records. Incomplete questionnaires were not taken into account for the analysis. The variables included in the analysis were:
- Patient baseline characteristics: Age, gender, procedure indication, previous history of inflammatory bowel disease (IBD), surgery, radiotherapy and drugs such as ASA or NSAIDs, symptoms prior CE procedure such as frequent bronchial aspirations, abdominal pain/discomfort, diarrhea, dysphagia and/or nausea/vomiting.
- CE procedure: Oesophageal, small bowel or colon.
- Type of AE: Capsule retention and location (esophagus, stomach, small bowel and colon), capsule aspiration and others.
- AEs-related symptoms: Abdominal pain/discomfort, abdominal distension, nausea, vomiting, cough, dyspnea and others.
- Previous gastrointestinal (GI) patency tests: Small bowel follow through, CT-scan, Patency© capsule and others.
- Clinical course/therapy: Spontaneous resolution, medical therapy, endoscopic therapy, surgery and others.
- Final diagnosis: NSAIDs stricture, tumor, IBD, idiopathic stricture and others.
- CE-related AEs considered in this study were: Capsule aspiration, capsule retention and CE-induced hemorrhage or perforation. Technical failures such as "end of battery life", "recording gaps" or illumination issues were not considered as CE-related AEs. Capsule retention was defined as a capsule remaining in the GI tract for 15 days or less if medical, endoscopic or surgical intervention had been initiated (14).
The clinical research ethics committee of each institution approved data compilation. Quantitative variables have been presented as mean [range] and qualitative variables as simple proportions. For comparison purposes, Pearson chi-square test was used for qualitative variables. P values under 0.05 were considered statistically significant. Data compilation and management has been performed using the SPSS version 15.0 (Chicago, Il).
Results
Data from 5,428 CE-procedures (63% males and 37% females; mean age 58.2 [15-82]) were included in the analysis. All CE-related AEs questionnaires were received according with the instructions given before the study.
CE-related AEs incidence
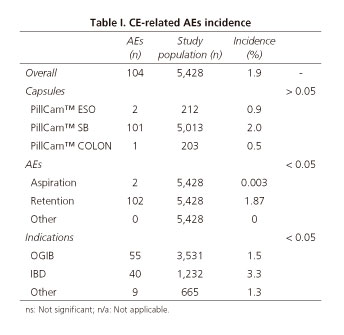
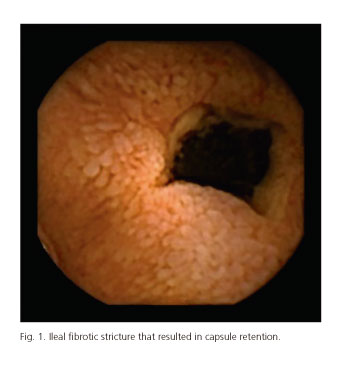
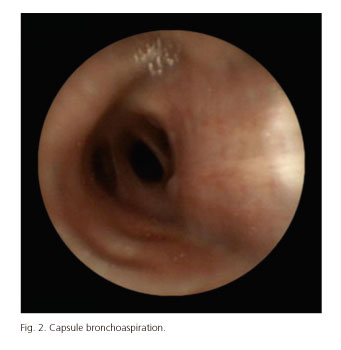
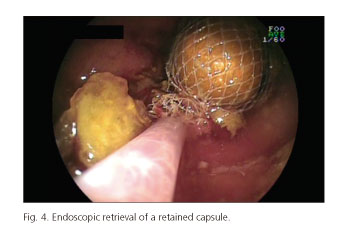
Table I shows CE-related AEs incidence. The overall incidence of CE-related AEs was 1.9% (104 out of 5,428 patients included in the analysis). Most of AEs were observed during small bowel CE (97% for small bowel, 1.7% for esophageal and 1.3% for colon CE). However, no statistically significant differences were observed in the incidence of AEs depending on the capsule used: 2.0%, 0.9% and 0.5% for small bowel, esophageal and colon CE, respectively, (p > 0.05). Capsule retention in the GI tract was the most frequent AE and its incidence was significantly higher than the one observed for capsule aspiration and other AEs (1.87% for capsule retention, 0.003% for capsule aspiration and 0% for other AEs). Focusing on the localization of capsule retention in the GI tract, small bowel was the most frequent site -88.2% (90/102) of cases- followed by esophagus (9/102; 8.8%), stomach (2/102; 1.9%) and colon (1/102; 0.9%). Most of the AEs were seen in patients with obscure gastrointestinal bleeding (OGIB) but the incidence was significantly higher in IBD patients: 1.5% and 3.3%, respectively (p < 0.05). Figures 1-4 show some cases of CE-related AEs.
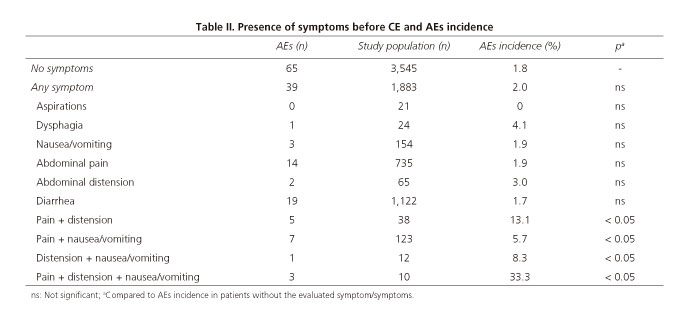
Symptoms before CE-related AEs
Table II shows the relationship between CE-related AEs incidence and symptoms before CE procedures.
CE-related AEs were more frequent in patients who had symptoms (frequent aspirations, dysphagia, nausea/vomiting, abdominal pain, abdominal distension and/or diarrhea), prior to capsule procedure. The incidence of capsule retention in the small bowel was significantly higher when the following combinations were observed before CE procedures: Abdominal pain and abdominal distension (13.1%), abdominal pain and nausea/vomiting (5.7%), abdominal distension and nausea/vomiting (8.3%) and abdominal pain, abdominal distension and nausea/vomiting (33.3%). The presence of 1 symptom alone or other combinations were not related with a higher or lower incidence of AEs. On the other hand, the presence other adverse events such as capsule aspiration or capsule retention outside the small bowel were not related to the presence of GI symptoms.
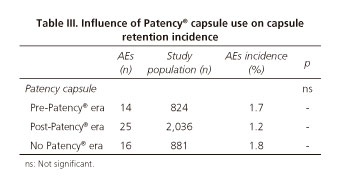
Influence of GI patency tests on CE-related AEs
All the information regarding the relationship between CE-related AEs and the use of GI patency tests is showed in tables III and IV.
Although no statistically significant differences were found, CE-related AEs incidence was higher in pre-Patency© capsule era and in those institutions that had never used the Patency© capsule than in post-Patency© capsule era: 1.7% (14/824) and 1.8% (16/881) versus 1.2% (25/2036), respectively (p > 0.05). The use of GI patency tests - except for Patency© capsule and MRI-enterography -before CE was not a good predictor for the presence of CE-related AEs. They were negative in 54.9% of capsule retentions. Capsule retention after a negative GI patency test procedure was significantly more frequent after small bowel follow through (SBFT) and abdominal CT-scan than after Patency© capsule and MRI-enterography: 1.9% for Patency© capsule, 0% for MRI, 21.5% for CT-scan and 34.3% for SBFT (p < 0.05).
Presence of symptoms during CE-related AEs
Symptoms (different from those suffered before CE procedure) related to CE AEs were absent in 64/104 (61.5%) of the cases. When present, patients suffered from abdominal pain in 25% (26/104) of cases, vomiting in 7.7% (8/104) of cases, abdominal distension in 1.9% (2/104) and other symptoms in 3.8% (4/104) of cases. The main 3 digestive symptoms (abdominal pain, distension and vomiting) were seen only in 2 out of 104 (1.9%) patients. Both patients finally underwent surgery where a small bowel stricture was confirmed. On the other hand, capsule aspiration was symptomatic in 50% of the cases (cough and moderate dyspnoea).
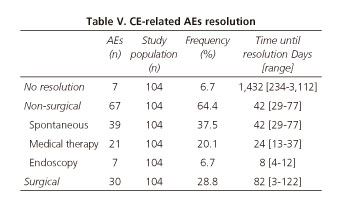
CE-related AEs resolution
Table V summarizes the outcome of CE-related AEs.
CE-AEs resolution was non-surgical in 64.4% of the cases: In 39 out of 104 (37.5%) patients was spontaneous, in 20 out of 104 (19.2%) was induced by medical therapy (steroids in 17 patients and laxatives in 3) and in 7 out of 104 (6.7%) was solved by endoscopy (3 by conventional digestive endoscopy, 3 by assisted-balloon enteroscopy and 1 by bronchoscopy). The mean time between CE-related AEs and resolution was 42 days (range 29-77 days) for spontaneous, 24 days (range 13-37 days) for medical therapy, 8 days (range 4-12 days) for endoscopic resolution and 82 days (range 3-122 days) for surgery. On the other hand, the mean time of capsule retention in those patients with no resolution was 1,432 days (range 234-3,112 days). All of them had a Crohn's disease and to date, they have not had symptoms related to capsule retention. Invasive capsule retrieval options were explained to all of them but all rejected, so they underwent medical therapy. This option has not been enough to solve the problem as the capsules have not been excreted.
CE-related AEs final diagnosis
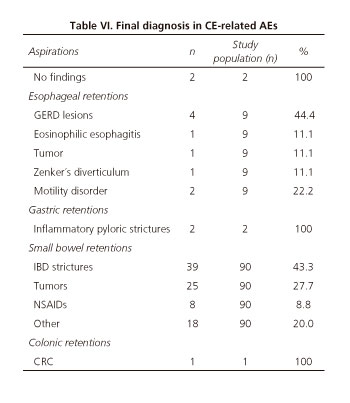
Table VI shows the final diagnosis of all CE-related AEs.
Those patients with capsule aspiration had no digestive or respiratory abnormalities that could have predicted the adverse event. The most frequent abnormality in all patients with esophageal capsule retention (n = 9) was the presence of inflammatory strictures (n = 5; peptic origin in 4 patients and eosinophilic origin in 1). All but one (1 peptic stricture that underwent esophageal capsule endoscopy for varices screening) had had an upper endoscopy prior to CE procedure with no significant esophageal lesions. However, all these examinations were performed more than 3 months before CE-procedures (14, 15, 20 and 22 weeks). There were 2 cases of capsule retention in the stomach and both were due to previous unreported pyloric inflammatory strictures (2 and 4 weeks). In both cases, the inflammatory nature of the strictures was confirmed by upper endoscopy and targeted biopsies. Most of the CE-related AEs were capsule retentions in the small bowel (90/104). The final diagnoses of these cases were as follows: IBD strictures in 39/90 cases (43.3%), tumors in 25/90 cases (27.7%), NSAIDs strictures in 8/90 cases (8.8%) and other in 18 cases (20.0%). Finally, the unique case of capsule retention in the colon was a small bowel capsule retained in a colorectal cancer. The patient was an elderly man who underwent small bowel capsule endoscopy because of severe anemia (he had had a normal colonoscopy 8 years before).
Discussion
Due to its advantages, capsule endoscopy is currently considered as a first line diagnostic tool for small bowel examination (3,4). However, although not critical, it has some limitations such as incomplete procedures or low accuracy in some clinical scenarios -i.e: small bowel tumors, hospitalized and diabetes patients, poor preparation...- and procedure-related AEs (2-4,6,15,16). Due to the low incidence of CE-related AEs, most cases have been published as case reports or small series (9-13). In fact, there is a lack of large series that could provide valuable information regarding real incidence, clinical outcome and management of CE-related AEs. The largest studies published of CE-related AEs report an overall incidence of capsule retention -no other complications were taken into account- of 1.4%, 1.3%, 2.5%, 1.4% and 1.9%, respectively (2,6,7,15,17). Our study reports the incidence of capsule retention but also the incidence of other CE-related AEs such as capsule aspiration. Some information included in this article has never been published before. The overall incidence of CE-related AEs in our study was 1.9% being 1.87% for capsule retention and 0.003% for capsule aspiration. No other CE-related AEs were found in our study population. The capsule retention incidence in our study is consistent with the previously mentioned articles, ranging between 1.3% and 2.5% (2,6,7,15,17). The incidence of capsule aspiration in a large cohort of patients has not been previously analysed. It has been reported only as case reports (9,13,18,19). Our analysis demonstrates that the incidence of capsule aspiration is very low. In fact, aspiration was observed only in 2 out of 5,428 patients resulting in an incidence of 0.003%. The absence of symptoms in capsule aspirations can be dangerous because the capsule can be retained in the airway until video visualization resulting in potential life-threating AEs including respiratory failure (19). Therefore, real time viewing, if possible, after capsule ingestion is highly recommended in elderly patients and in those situations where capsule swallowing has been difficult or symptomatic. Capsule aspiration should be considered an emergency. The presence of dysphagia is a relative contraindication for capsule endoscopy. However, 24 out of 5,428 patients included in the present study had dysphagia and only 1 of them suffered from a CE-related complication but it was not an esophageal retention or capsule bronchoaspiration. This patient suffered from a capsule retention in the small bowel. The presence of dysplagia was not correlated with esophageal retention or capsule aspiration in our study but since most of patients who undergo capsule endoscopy have previously undergone upper endoscopy, it is very unfrequent to see this complication in these patients (0% in our study). On the other hand, in those patients with dysphagia and at risk of proximal esophageal retention without organic obstructive lesions (i.e. Zenker's diverticulum or esophageal motility disorder) or unable to swallow the capsule, it may be placed endoscopically in the stomach/duodenum. This eliminates the risk of esophageal capsule retention, delays and/or bronchoaspiration. According to the capsule used, there were no significant differences in the incidence of CE-related AEs in those patients who underwent esophageal, small bowel or colon capsule endoscopy. Esophageal and colon capsule endoscopy showed slightly lower AE incidences, which could be explained by patients' clinical profile. In fact, the main indications for both procedures were esophageal varices, gastroesophageal reflux disease and colonic polyps that are usually non-obstructive lesions. On the other hand, small bowel lesions are usually not expected in these settings. Previous large reported series of esophageal and colon capsule endoscopy showed a similar CE-related AEs incidence (20-22). Regarding procedure indications, the incidence of AEs was significantly higher in IBD than in OGIB patients (3.3% versus 1.5%, respectively) being capsule retention in the small bowel the event most frequently observed. This information is consistent with that previously reported in most papers where the incidence in OGIB and IBD is up to 2% and 4%, respectively (2,6,7,15,17). As recommended by current guidelines (23), all patients undergoing small bowel capsule endoscopy due to OGIB should firstly undergo upper and lower GI endoscopy. However, there are some clinical scenarios that make physicians individualize diagnostic approaches avoiding upper and/or lower GI endoscopy. This strategy may result in a higher risk, although very low, of capsule retention outside the small bowel -esophagus, stomach or colon- as occurred with one of our patients. Based on previous reports (6,17,24,25), the incidence of capsule retention is significantly higher in known than in suspected Crohn's disease (up to 13% and 5%, respectively). In fact, while most of capsule endoscopy procedures are positive for lesions in those patients with known Crohn's disease, more than the 50% of procedures will be negative in those patients with suspected Crohn's disease (26-28). On the other hand, in those patients with symptoms suggesting bowel obstruction, the incidence of capsule retention can raise up to 20% (8). In fact, as demonstrated by our paper, the presence of a combination of symptoms such as abdominal pain and distension, abdominal pain and nausea/vomiting and abdominal distension and nausea/vomiting is related with a significantly higher incidence of capsule retention. Based on our results, the presence of abdominal distension, abdominal pain and/or nausea/vomiting alone were not at risk of capsule retention (3.0%, 1.9% and 1.9%, respectively). All of them underwent at least one GI patency test, mostly SBFT and CT scan that were not able to predict the complication. Therefore, it is recommended to take a complete clinical history and to undergo Patency® capsule when abdominal symptoms are present. Other modalities for GI patency testing such as abdominal CT and SBFT are not accurate enough for stricture detection (6,8). Our results are in accordance with these studies and showed that 25.5% and 34.3% of patients with capsule retention had a previous negative abdominal CT and SBFT, respectively. Hence, a negative radiologic examination, except for Patency test and MRI-enterography, do not exclude from the risk of capsule retention. The performance of GI patency tests in patients with and without complications was not directly compared since participants were not asked to compile the data from the no-complications group (more than 5,000 patients). This could be very interesting in order to evaluate if the incidence of complications could be affected by the performance of a GI patency test. More than half patients with negative GI patency test had a complication. This means that it is unlikely that the SBFT and CT scan could have any influence on the incidence of CE-related complications (i.e. capsule retention). The percentage of patients with negative MRI-enterography and a CE-related complication was 0%. However, this study was designed in 2008 and started in 2009. The availability of the MRI-enterography was very low and we do not know the number of procedures performed in our patients (probably low). So conclusions with MRI-enterography should be taken into account carefully. The role of MRI in predicting capsule retention in the small bowel is still unclear, as it seems that it sometimes overstages and loses small bowel strictures. In fact, some false negatives and positives of MRI have been recently reported (29,30). On the other hand, capsule retention after a negative capsule patency examination is rare as showed by our paper and other articles (31-33). This clearly indicates that the patency capsule should be the method of choice to evaluate the patency of the GI tract. Our study demonstrates that the incidence CE-AEs is lower since the introduction of Patency© capsule. A recent paper showed also that the combination of Patency capsule and abdominal ultrasonography appears to be a good indicator for the risk of capsule retention (34).
Regarding symptoms during AEs, CE-related AEs were asymptomatic in more than 60% of the cases. Symptoms, when present, were usually mild and did not require aggressive therapeutic maneuvers. In fact, only patients with the combination of abdominal pain, distension and nausea/vomiting (2 out of 104 patients; 1.9%) that meant bowel obstruction underwent surgery. Two thirds of the AEs reported in our study were managed by non-surgical methods and no AE-related deaths were observed. Surprisingly, surgery was the therapy of choice, even in asymptomatic cases, in most of the largest CE-related AEs series ranging from 53.1% to 92.8% (2,6,7,15,17). Based on our results, capsule retention can be considered a non-emergent event that permits waiting for the best therapeutic solution. This should not be considered a risky attitude since the incidence of acute symptoms is very low. In fact, as reported, the capsule can remain inside the small bowel without causing symptoms for several years (17,35,36). As demonstrated in one-third of our patients and in some published cases, another good reason to the "wait and see" management is that some capsules can be naturally excreted during follow-up (6,17,35). This has an explanation: Some capsules could be temporarily retained in diverticula or in strictures with a high inflammatory component that may improve during the follow-up spontaneously. Apart from the "wait and see" option, medical therapy based on steroids and/or laxatives or endoscopic retrieval of the capsule could be other feasible and effective therapeutic approaches (2,6,7,15,17). Anyway, surgery should be the last therapeutic option unless the origin is a malignant lesion or the patient develops acute small bowel obstruction symptoms.
The main limitations of this study are the retrospective design of the analysis and the lack of an objective consensus regarding capsule retention. In fact, the present study is retrospective and this may result in some difficulties in data collection resulting in missed information. To simplify data collection, a simple questionnaire with standardized questions and answers containing the target information was designed and sent to all participants. Maybe we could obtain more information developing a more complex questionnaire but it could result in missed cases. Anyway, all participants were asked to make all efforts in searching for the requested information in databases and/or medical records. Although incomplete questionnaires were not supposed to be taken into account, all received forms were complete. This is probably due to the fact that CE-related AEs are not frequent and usually are well documented. However, the large sample size used in this study and the accuracy and completeness of data collection minimizes the negative effect of possible bias.
In summary, the incidence of CE-related AEs is very low being the capsule retention the most frequent AE. A complete medical history is mandatory prior to CE and in those cases with abdominal symptoms, the Patency© capsule can help to predict the integrity of the GI tract reducing the incidence of capsule retention. Capsule aspiration is exceptional should be considered an emergency and capsule retention without acute obstructive symptoms should be managed conservatively whenever possible.
References
1. Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. DOI: 10.1038/35013140. [ Links ]
2. Liao Z, Gao R, Xu C, Li, et al. Indications and detection, completion and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest Endosc 2010;71:280-6. DOI: 10.1016/j.gie.2009.09.031. [ Links ]
3. Neumann H, Fry LC, Neurath MF. Review article on current applications and future concepts of capsule endoscopy. Digestion 2013;87:91-9. DOI: 10.1159/000345346. [ Links ]
4. Hale MF, Sidhu R, McAlindon ME. Capsule endoscopy: Current practice and future directions. World J Gastroenterol 2014;20:7752-9. DOI: 10.3748/wjg.v20.i24.7752. [ Links ]
5. Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 2005;3:133-41. DOI: 10.1016/S1542-3565(04)00619-6. [ Links ]
6. Cheon JH, Kim YS, Lee IS, et al.; Korean Gut Image Study Group. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy 2007;39:1046-52. DOI: 10.1055/s-2007-966978. [ Links ]
7. Li F, Gurudu SR, De Petris G, et al. Retention of the capsule endoscope: A single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc 2008;68:174-80. DOI: 10.1016/j.gie.2008.02.037. [ Links ]
8. Cheifetz AS, Lewis BS. Capsule endoscopy retention: Is it a complication? J Clin Gastroenterol 2006;40:688-91. DOI: 10.1097/00004836-200609000-00005. [ Links ]
9. Nathan SR, Biernat L. Aspiration - an important complication of small-bowel video capsule endoscopy. Endoscopy 2007;39(Supl. 1):E343. DOI: 10.1055/s-2007-995327. [ Links ]
10. Um S, Poblete H, Zavotsky J. Small bowel perforation caused by an impacted endocapsule. Endoscopy 2008;40(Supl. 2):E122-3. DOI: 10.1055/s-2007-995694. [ Links ]
11. Tanaka Y, Motomura Y, Akahoshi K, et al. Capsule endoscopic detection of bleeding Meckel's diverticulum, with capsule retention in the diverticulum. Endoscopy 2010;42(Supl. 2):E199-200. DOI: 10.1055/s-0030-1255696. [ Links ]
12. Ferreira F, Bastos P, Cardoso H, et al. Retention of endoscopic capsule in an umbilical hernia. 19. Endoscopy 2011;43(Supl. 2) UCTN:E111-2. DOI: 10.1055/s-0030-1256141. [ Links ]
13. Ding NS, Hair C, De Cruz P, et al. Education and Imaging. Gastrointestinal: Symptomatic bronchial aspiration of capsule endoscope - a significant complication. J Gastroenterol Hepatol 2013;28:761. DOI: 10.1111/jgh.12173. [ Links ]
14. Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy 2005;37:1065-7. DOI: 10.1055/s-2005-870264. [ Links ]
15. Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations and failures of capsule endoscopy: A review of 733 cases. Gastrointest Endosc 2005;62:712-6. DOI: 10.1016/j.gie.2005.05.002. [ Links ]
16. Rondonotti E, Villa F, Mulder CJJ, et al. Small bowel capsule endoscopy in 2007: Indications, risks and limitations. World J Gastroenterol 2007;13:6140-9. DOI: 10.3748/wjg.13.6140. [ Links ]
17. Höög CM, Bark LA, Arkani J, et al. Capsule retentions and incomplete capsule endoscopy examinations: An analysis of 2300 examinations. Gastroenterol Res Pract 2012;2012:518718. DOI: 10.1155/2012/518718. [ Links ]
18. Pezzoli A, Fusetti N, Carella A, et al. Asymptomatic bronchial aspiration and prolonged retention of a capsule endoscope: A case report. J Med Case Rep 2011;5:341. DOI: 10.1186/1752-1947-5-341. [ Links ]
19. Girdhar A, Usman F, Bajwa A. Aspiration of capsule endoscope and successful bronchoscopic extraction. J Bronchology Interv Pulmonol 2012;19:328-31. DOI: 10.1097/LBR.0b013e31826e3b53. [ Links ]
20. de Franchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology 2008;47:1595-603. DOI: 10.1002/hep.22227. [ Links ]
21. Van Gossum A, Munoz Navas M, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264-70. DOI: 10.1056/NEJMoa0806347. [ Links ]
22. Bhardwaj A, Hollenbeak CS, Pooran N. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barret's esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol 2009;104:1533-9. DOI: 10.1038/ajg.2009.86. [ Links ]
23. Ladas SD, Triantafyllou K, Spada C, et al.; ESGE Clinical Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE): Recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy 2010;42:220-7. DOI: 10.1055/s-0029-1243968. [ Links ]
24. Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule in patients with known or suspected Crohn's disease. Am J Gastroenterol 2006;101:2218-22. DOI: 10.1111/j.1572-0241.2006.00761.x. [ Links ]
25. Cotter J, Castro FD, Moreira MJ, et al. Tailoring Crohn's disease tretament: The impact of small bowel capsule endoscopy. J Crohns Colitis 2014;8:1610-5. DOI: 10.1016/j.crohns.2014.02.018. [ Links ]
26. Herrerías JM, Caunedo A, Rodríguez-Téllez M, et al. Capsule endoscopy in patients with suspected Crohn's disease and negative endoscopy. Endoscopy 2003;35:564-8. DOI: 10.1055/s-2003-40241. [ Links ]
27. Girelli CM, Porta P, Malacrida V, et al. Clinical outcome of patients examined by capsule endoscopy for suspected small bowel Crohn's disease. Dig Liver Dis 2007;39:148-54. DOI: 10.1016/j.dld.2006.10.018. [ Links ]
28. Arguelles-Arias F, Rodriguez-Oballe J, Duarte-Chang C, et al. Capsule endoscopy in small bowel Crohn's disease. Gastroenterol Res Pract 2014;2014:529136. DOI: 10.1155/2014/529136. [ Links ]
29. Skovsen AP, Burchart J, Burgdorf SK. Capsule endoscopy: A cause of late small bowel obstruction and perforation. Case Rep Surg 2013;2013:458108. DOI: 10.1155/2013/458108. [ Links ]
30. Wiarda BM, Mensink PBF, Heine DGN, et al. Small bowel Crohn's disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdom Imaging 2012;37:397-408. DOI: 10.1007/s00261-011-9816-8. [ Links ]
31. Spada C, Riccioni ME, Costamagna G. The new, dissolving patency capsule: A safe and effective tool to avoid the complication of retained video capsules. J Clin Gastroenterol 2008;42:761-2. DOI: 10.1097/MCG.0b013e31802e7f11. [ Links ]
32. Herrerias JM, Leighton JA, Costamagna G, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc 2008;67:902-9. DOI: 10.1016/j.gie.2007.10.063. [ Links ]
33. Postgate AJ, Burling D, Gupta A, et al. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: A 3-year technical review. Dig Dis Sci 2008;53:2732-8. DOI: 10.1007/s10620-008-0210-5. [ Links ]
34. Shiotani A, Hata J, Manabe N, et al. Clinical relevance of patency capsule combined with abdominal ultrasonography to detect small bowel strictures. Eur J Gastroenterol Hepatol 2014;26:1434-8. DOI: 10.1097/MEG.0000000000000225. [ Links ]
35. Sears DM, Avots-Avontins A, Culp K, et al. Frequency and clinical outcome of capsule retention during capsule endoscopy for GI bleeding of obscure origin. Gastrointest Endosc 2004;60:822-7. DOI: 10.1016/S0016-5107(04)02019-X. [ Links ]
36. Harrington C, Rodgers C. The longest duration of retention of a video capsule. BMJ Case Rep 2014;8;2014. DOI: 10.1136/bcr-2013-203241. [ Links ]
![]() Correspondence:
Correspondence:
Ignacio Fernández-Urien.
Department of Gastroenterology.
Complejo Hospital de Navarra.
C/ Irunlarrea, 3.
31008 Pamplona, Navarra. Spain
e-mail: ifurien@yahoo.es
Received: 23-04-2015
Accepted: 08-09-2015