Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.8 Madrid ago. 2016
https://dx.doi.org/10.17235/reed.2016.4474/2016
Agenesis of the dorsal pancreas: systematic review of a clinical challenge
Javier A. Cienfuegos1, Fernando Rotellar1, Joseba Salguero1, Alberto Benito2, José Luis Solórzano3 and Bruno Sangro4
Departments of 1General Surgery, 2Radiology, 3Pathology, and
4Hepatology Unit - Department of Internal Medicine. Clínica Universidad de Navarra. Facultad de Medicina. Universidad de Navarra. Pamplona, Spain
ABSTRACT
Background: Agenesis of the dorsal pancreas is a rare malformation. Since 1911 and until 2008, 53 cases have been reported. Several authors have recently described the association of this anomaly with neoplasia of the ventral pancreas, thus we performed a systematic review of the literature from 2008 to 2015.
Methods: A systematic review of the Medline and ISI Web of Science Databases from 2008 until 2015 was carried out, and 30 articles which met the inclusion criteria were identified that included a total of 53 patients: 7 children and 46 adults.
Conclusions: Although dorsal pancreatic agenesis is a rare malformation, given its association with non-alcoholic pancreatitis and neoplasia of the residual pancreas, physicians should maintain an expectant attitude.
Key words: Dorsal agenesis of the pancreas. Short pancreas. Diabetes mellitus. Mucinous cyst. Pancreas anomaly.
Introduction
Dorsal pancreatic agenesis (DPA) is a very rare malformation which consists of the absence of the neck, body and tail of the pancreas derived from the bud of the dorsal endoderm (1,2). This anomaly was first described in 1911, and 53 cases were reported up to 2008 (3,4). Several authors have reported an association between DPA and acinar-ductal metaplasia (ADM), and neoplasia of the remnant pancreas (ventral pancreas) and diabetes (5-9).
The purpose of this systematic review was to evaluate the clinical features and pathologic findings of patients with DPA following on from seminal study by Schnedl (4).
Methods
Search strategy
We searched the medical literature using a comprehensive text word and MESH-based electronic search of MEDLINE and EMBASE; the Pub Med database and Scopus was queried including the terms: dorsal pancreatic agenesis, short pancreas, pancreatic agenesis, pancreatic hypoplasia, pancreatic anomaly and pancreatic aplasia from 1998 until December 2015, excluding all the cases previously reported by Schnedl (4). Additionally, the references of relevant articles were hand searched for additional material. The search included abstracts, case-series, case reports, editorials, letters to the editor, and images in gastroenterology. We excluded review articles, studies reporting on fetuses, research animals, those in which clinical and pathologic data were missing, letters related to reported cases or those where the full text or abstract was not written in English, Spanish, French or German. The search was performed by the two authors -JAC and JS- and the results were contrasted with one of the senior authors (FR).
Eligibility criteria and data collection
All reports on DPA were included, which described at least 1 case. Full-text articles were obtained to clarify the potential eligibility and the selected studies were evaluated in accordance with the Preferred reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement (10). Two authors (JAC, FR) independently assessed the studies selected and extracted the following data: first author, year of publication, country of origin, number of patients, age, gender, type of clinical presentation, associated disorders, genetic analyses, radiologic characteristics, treatment undertaken and pathology report.
Results
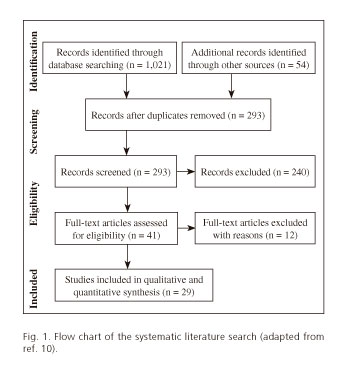
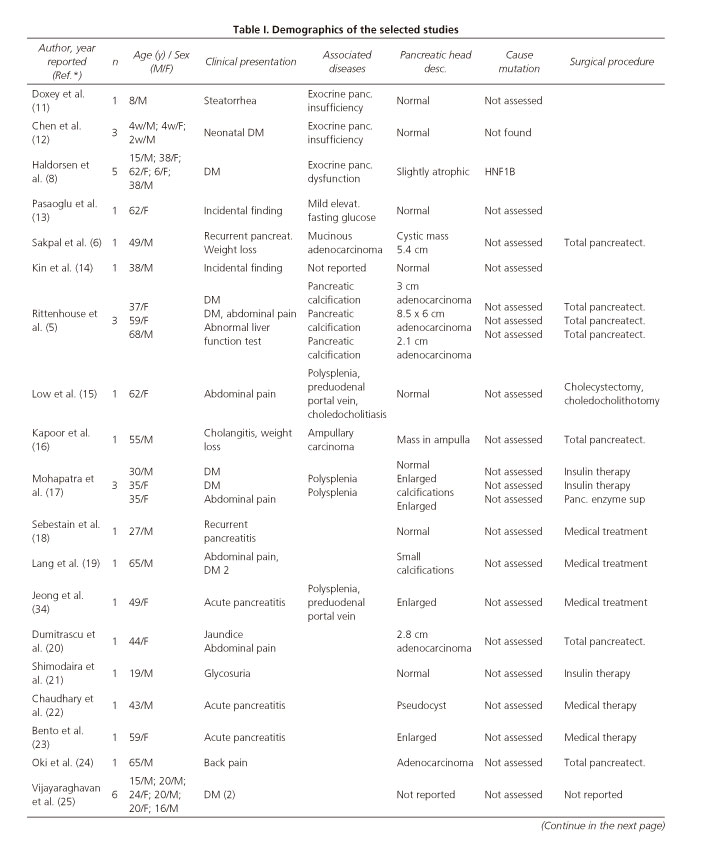
In total 1,021 articles were retrieved from the primary database search and 54 articles were retrieved by manual cross-search of the reference list. Following removal of duplicates, 293 articles were assessed for further analysis; screening of the titles and abstracts enabled us to exclude 240 articles. The full-text articles of the remaining 41 studies were assessed for eligibility; we then excluded all reviews, commentaries on published cases and those studies not meeting the requirements for DPA: short pancreas or pancreatic hypoplasia. We excluded the cases of complete agenesia of the pancreas (3 articles), the so-called short pancreas (5 articles) and others reported as atrophy of the body and tail. A flow chart of the literature search is shown in figure 1. Overall 30 articles met the inclusion criteria and these reported on 53 patients; 7 children and 46 adults. The disorders associated with the 53 cases reported since 2008 are summarized in table I (5-9,11-34).
Abdominal pain and diabetes mellitus were the most frequent clinical manifestation in 10 patients. Nine of the 53 patients underwent total pancreatectomy mainly for adenocarcinoma of the pancreas (Table I). Notably 4 cases were incidentally diagnosed.
Discussion
DPA is a rare malformation caused by the absence of the dorsal pancreatic bud of the endoderm (1,35,36). The entity was first described by Heiberg in 1911 (3) and since then until 2008 53 cases have been reported by Schnedl (4) in 2009. From 2008 to 2015, we have identified 53 cases, 7 in children and 46 in adults. The high number of cases reported (53) in the last 8 years merits special mention, especially as in the previous 100 years (1911-2008) Schnedl only identified 53 cases. This may be due to the greater accessibility to imaging techniques.
The clinical manifestations of DPA reflect the abnormalities in the embryogenesis of the dorsal pancreas. The exocrine and endocrine tissues of the pancreas come from a common pool of multipotent progenitor cells from the foregut which give rise to the trachea, lungs, esophagus, stomach, thyroid, liver, bile ducts and the pancreas (36-38).
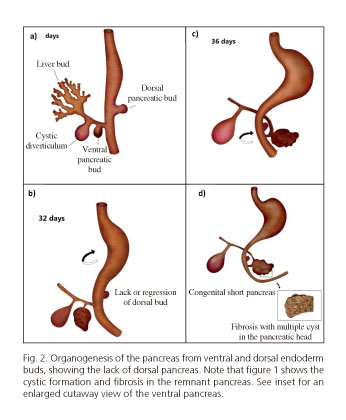
In humans the initial bud of the pancreas is produced on day 26 of gestation (G 26d) with the dorsal bud and the two ventral buds forming on day 30 (G 30d) (Fig. 2). The left ventral bud regresses whilst the right ventral bud migrates posteriorly together with the rotation of the gut at 6-7 weeks of gestation (G 6-7w) and fuses with the dorsal bud. With the fusion of both buds, morphogenesis and differentiation begin in the acinar, ductal and endocrine cells (1,35,37,38).
The ventral and dorsal buds share specific signaling pathways and transcription factors (TF) such as Hedgehog, via Notch or TF: fibroblast growth factor-10 (FGF-10), epidermal growth factor (EGF), retinoic acid, Wnt signaling and bone morphogenetic protein (BMP) produced by the surrounding mesoderm (39).
The transition from multipotent progenitor cells in an organ with exocrine secreting units and endocrine tissue is a strictly regulated multi-sequential process. As pancreatic morphogenesis progresses, the TF are more selective for the 3 cell lines: bHLH neurogenin 3 (Ngn3) for β cells, SOX9 for ductal cells and PTF1 for acinar cells.
A detailed description of pancreatic embryogenesis is beyond the scope of this study and several excellent reviews are available (1,35-37,40-42).
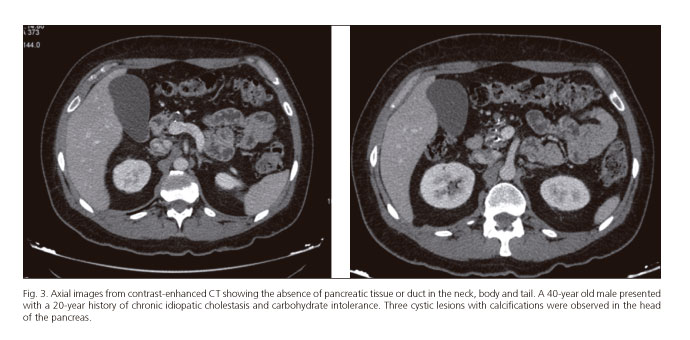
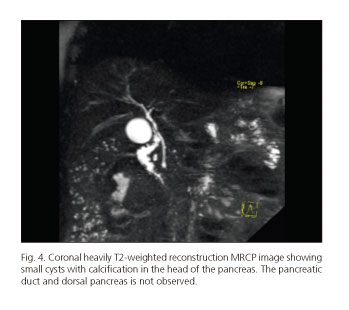
The clinical presentation of DPA varies greatly ranging from incidental detection on X-ray, surgery or autopsy through to the development of a ductal adenocarcinoma of the pancreas (4,5). Diagnosis requires confirmation of the absence of the neck, body and tail of the pancreas and duct of Wirsung using endoscopic retrograde cholangiopancreatography (ERCP) or MRCP (Figs. 3 and 4). DPA has been reported both in children and adults and may be associated with an autosomal dominant mutation of the hepatocyte nuclear factor 1B (HNF1B) gene, although in most cases it is sporadic (43-45).
Abdominal pain and diabetes are the most frequent clinical manifestations reflecting exocrine and endocrine insufficiency as most of the islands of Langerhans are located in the tail of the pancreas (4,19,46,47).
The association between cystic lesions and non-alcoholic chronic pancreatitis with pancreatic ductal adenocarcinoma (PDA) has recently been highlighted by Rittenhouse et al. (5), in 3 patients who also presented dorsal pancreas agenesia. Other authors have reported an association with ampullary adenocarcinoma and intraductal papillary mucinous neoplasm (IPMN) (6,26). There have also been reports of an increase in the size of the remnant pancreas and recurrent acute pancreatitis as a form of presentation (48).
These findings confirm the development of phenomena related to a reparative response in the residual pancreas. In experimental models of acute pancreatitis and in partial resections of the pancreas, regeneration of the pancreas has been reported in mice and children with nesidioblastosis, but never in adults (41,48,49). In the regenerative response of the pancreas, the presence of acinar-ductal metaplasia has been reported where there is an upregulation of ductal markers such as CK19 and SOX9. In mouse models, the development of PDA with a ductal phenotype has been confirmed. This is consistent with the clinical findings of Rittenhouse (5,50).
Conclusion
Although DPA is a very rare malformation, given its association with non-alcoholic chronic pancreatitis and neoplasms of the residual pancreas (the ventral pancreas), physicians should maintain an expectant attitude.
References
1. Shih HP, Wang A, Sander M. Pancreas organogenesis: From lineage determination to morphogenesis. Annu Rev Cell Dev Biol 2013;29:81-105. DOI: 10.1146/annurev-cellbio-101512-122405. [ Links ]
2. Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev Dyn 2011;240(3):530-65. DOI: 10.1002/dvdy.22584. [ Links ]
3. Heiberg KA. Ein Fall von fehlender Cauda pancreatis (bei einem Diabetiker). Centralbl Allg Pathol Patholog Anat 1911;22:676-7. [ Links ]
4. Schnedl WJ, Piswanger-Soelkner C, Wallner SJ, et al. Agenesis of the dorsal pancreas and associated diseases. Dig Dis Sci 2009;54(3):481-7. DOI: 10.1007/s10620-008-0370-3. [ Links ]
5. Rittenhouse DW, Kennedy EP, Mascaro AA, et al. The novel triad of dorsal agenesis of the pancreas with concurrent pancreatic ductal adenocarcinoma and nonalcoholic chronic calcific pancreatitis: A case series and review of the literature. J Gastrointest Surg 2011;15(9):1643-9. DOI: 10.1007/s11605-011-1542-6. [ Links ]
6. Sakpal SV, Sexcius L, Babel N, et al. Agenesis of the dorsal pancreas and its association with pancreatic tumors. Pancreas 2009;38(4):367-73. DOI: 10.1097/MPA.0b013e318196c401. [ Links ]
7. Mistry JH, Yadav A, Nundy S. Ampullary carcinoma in a patient with agenesis of the dorsal pancreas: A case report. Indian J Surg 2015;77(Suppl. 1):32-4. DOI: 10.1007/s12262-014-1082-x. [ Links ]
8. Haldorsen IS, Vesterhus M, Raeder H, et al. Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet Med 2008;25(7):782-7. DOI: 10.1111/j.1464-5491.2008.02460.x. [ Links ]
9. Cho HS, Woo JY, Hong HS, et al. Morphologic classification of congenital short pancreas on multidetector computed tomography. J Comput Assist Tomogr 2013;37(5):797-804. DOI: 10.1097/RCT.0b013e31829ce256. [ Links ]
10. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6(7):e1000100. DOI: 10.1371/journal.pmed.1000100. [ Links ]
11. Doxey BW, Jackson WD, Adler DG. A unique presentation: Dorsal agenesis of the pancreas manifesting as pancreatic exocrine insufficiency in the absence of diabetes mellitus in an 8-year-old boy. Dig Dis Sci 2008;53(7):2005-6. DOI: 10.1007/s10620-007-0094-9. [ Links ]
12. Chen R, Hussain K, Al-Ali M, et al. Neonatal and late-onset diabetes mellitus caused by failure of pancreatic development: Report of 4 more cases and a review of the literature. Pediatrics 2008;121(6):e1541-7. DOI: 10.1542/peds.2007-3543. [ Links ]
13. Pasaoglu L, Vural M, Hatipoglu HG, et al. Agenesis of the dorsal pancreas. World J Gastroenterol 2008;14(18):2915-6. DOI: 10.3748/wjg.14.2915. [ Links ]
14. Kin T, Shapiro J. Partial dorsal agenesis accompanied with circumportal pancreas in a donor for islet transplantation. Islets 2010;2(3):146-8. DOI: 10.4161/isl.2.3.11715. [ Links ]
15. Low JP, Williams D, Chaganti JR. Polysplenia syndrome with agenesis of the dorsal pancreas and preduodenal portal vein presenting with obstructive jaundice - A case report and literature review. Br J Radiol 2011;84(1007):e217-20. DOI: 10.1259/bjr/27680217. [ Links ]
16. Kapoor A, Singh R. Periampullary carcinoma in a patient with agenesis of dorsal pancreas. J Surg Case Rep 2011;2011(9):4. [ Links ]
17. Mohapatra M, Mishra S, Dalai PC, et al. Imaging findings in agenesis of the dorsal pancreas. Report of three cases. JOP 2012;13(1):108-14. [ Links ]
18. Sebestain S. Congenital short pancreas. Trop Gastroenterol 2012; 33(4):283-5. DOI: 10.7869/tg.2012.72. [ Links ]
19. Lang K, Lasson A, Muller MF, et al. Dorsal agenesis of the pancreas - A rare cause of abdominal pain and insulin-dependent diabetes. Acta Radiol 2012;53(1):2-4. DOI: 10.1258/ar.2011.110480. [ Links ]
20. Dumitrascu T, Scarlat A, Diaconescu A, et al. Dorsal pancreas agenesis and ductal adenocarcinoma: Surgical implications of an extremely rare association. Chirurgia (Bucur) 2012;107(3):389-92. [ Links ]
21. Shimodaira M, Kumagai N, Sorimachi E, et al. Agenesis of the dorsal pancreas: A rare cause of diabetes. Intern Emerg Med 2012;7(1):83-4. DOI: 10.1007/s11739-011-0620-9. [ Links ]
22. Chaudhary P, Arora MP. Agenesis of dorsal pancreas with eventration of diaphragm and intrapancreatic pseudocyst: A rare entity. Clin Pract 2013;3(1):e7. DOI: 10.4081/cp.2013.e7. [ Links ]
23. Bento A, Baptista H, Oliveira F. Congenital pancreas malformations: A clinical case report. Rev Assoc Med Bras 2013;59(1):35-9. DOI: 10.1016/S2255-4823(13)70427-2. [ Links ]
24. Oki Y, Onoyama H, Nikaido M, et al. Pancreatic cancer in a patient with congenital agenesis of the dorsal pancreas. Nihon Shokakibyo Gakkai Zasshi 2013;110(6):1044-53. [ Links ]
25. Vijayaraghavan SB, Gouru S, Senthil S. Sonographic features of agenesis of dorsal pancreas. Indian J Radiol Imaging 2013;23(2):179-82. DOI: 10.4103/0971-3026.116570. [ Links ]
26. Sannappa RM, Buragohain J, Sarma D, et al. Agenesis of dorsal pancreas associated with periampullary pancreaticobiliary type adenocarcinoma. JOP 2014;15(5):489-92. [ Links ]
27. Zhou Y, Chen M, Liu Y. Agenesis of dorsal pancreas confirmed by three-dimensional reconstruction CT. Int J Clin Exp Med 2014; 7(9):3110-2. [ Links ]
28. Lim CC, Lai AH, Choo JC. Asymptomatic proteinuria, renal cysts and dorsal pancreas agenesis. Clin Kidney J 2014;7(4):411-2. DOI: 10.1093/ckj/sfu066. [ Links ]
29. Thakur S, Jhobta A, Sharma D, et al. MR in complete dorsal pancreatic agenesis: Case report and review of literature. Indian J Radiol Imaging 2014;24(2):156-9. DOI: 10.4103/0971-3026.134401. [ Links ]
30. Lal A, Pavunesan SK, Mahalingam H, et al. A triad of complete dorsal pancreatic agenesis, pancake kidney and bicornuate uterus. An association or an incidental finding: First case in literature. JOP 2015;16(2):189-91. [ Links ]
31. Kumar R, Vyas K, Agrahari N, et al. Complete agenesis of the dorsal pancreas: Case report with imaging findings and review of the literature. Malawi Med J 2015;27(2):73-4. [ Links ]
32. Sahoo RK, Dhae SK, Behera P. Dorsal pancreas agenesis - A rare case report. Eur J Anat 2015;19(3):291-3. [ Links ]
33. Guimaraes AB, Guimaraes CA, Manso JE. Agenesis or pseudoagenesis of the dorsal pancreas. Rev Col Bras Cir 2015;42(5):352-5. [ Links ]
34. Jeong JH, Kim GH, Song GA, et al. Polysplenia syndrome with congenital agenesis of dorsal pancreas presenting as acute pancreatitis and the role of endoscopic ultrasonography in its diagnosis. Korean J Gastroenterol 2012;60(1):47-51. DOI: 10.4166/kjg.2012.60.1.47. [ Links ]
35. Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology 2007;132(2):745-62. DOI: 10.1053/j.gastro.2006.12.054. [ Links ]
36. Tadokoro H, Takase M, Nobukawa B. Development and congenital anomalies of the pancreas. Anat Res Int 2011;2011:351217. DOI: 10.1155/2011/351217. [ Links ]
37. Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes 2014;21(2):77-82. DOI: 10.1097/MED.0000000000000047. [ Links ]
38. Gittes GK. Developmental biology of the pancreas: A comprehensive review. Dev Biol 2009;326(1):4-35. DOI: 10.1016/j.ydbio.2008.10.024. [ Links ]
39. Kumar M, Melton D. Pancreas specification: A budding question. Curr Opin Genet Dev 2003;13(4):401-7. DOI: 10.1016/S0959-437X(03)00089-3. [ Links ]
40. De Vas MG, Kopp JL, Heliot C, et al. Hnf1b controls pancreas morphogenesis and the generation of Ngn3+ endocrine progenitors. Development 2015;142(5):871-82. DOI: 10.1242/dev.110759. [ Links ]
41. Cleveland MH, Sawyer JM, Afelik S, et al. Exocrine ontogenies: On the development of pancreatic acinar, ductal and centroacinar cells. Semin Cell Dev Biol 2012;23(6):711-9. DOI: 10.1016/j.semcdb.2012.06.008. [ Links ]
42. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 2009;25:221-51. DOI: 10.1146/annurev.cellbio.042308.113344. [ Links ]
43. Schnedl WJ, Reisinger EC, Schreiber F, et al. Complete and partial agenesis of the dorsal pancreas within one family. Gastrointest Endosc 1995;42(5):485-7. DOI: 10.1016/S0016-5107(95)70055-2. [ Links ]
44. Haldorsen IS, Raeder H, Vesterhus M, et al. The role of pancreatic imaging in monogenic diabetes mellitus. Nat Rev Endocrinol 2011;8(3):148-59. DOI: 10.1038/nrendo.2011.197. [ Links ]
45. Body-Bechou D, Loget P, D'Herve D, et al. TCF2/HNF-1beta mutations: 3 cases of fetal severe pancreatic agenesis or hypoplasia and multicystic renal dysplasia. Prenat Diagn 2014;34(1):90-3. DOI: 10.1002/pd.4264. [ Links ]
46. Dor Y, Brown J, Martínez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429(6987):41-6. DOI: 10.1038/nature02520. [ Links ]
47. Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 2015; 5:14634. DOI: 10.1038/srep14634. [ Links ]
48. Murtaugh LC, Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol 2015;77:229-49. DOI: 10.1146/annurev-physiol-021014-071727. [ Links ]
49. Menge BA, Tannapfel A, Belyaev O, et al. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes 2008;57(1):142-9. DOI: 10.2337/db07-1294. [ Links ]
50. Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010;138(3):1166-77. DOI: 10.1053/j.gastro.2009.12.005. [ Links ]
![]() Correspondence:
Correspondence:
Javier A. Cienfuegos.
Department of General Surgery.
Clínica Universidad de Navarra.
Universidad de Navarra.
Avda. Pío XII, 36.
31008 Pamplona, Spain
e-mail: fjacien@unav.es
Received: 03-06-2016
Accepted: 03-06-2016