Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.12 Madrid dic. 2017
https://dx.doi.org/10.17235/reed.2017.4449/2016
Comparison of esophageal motility in gastroesophageal reflux disease with and without globus sensation
Yuming Tang, Jia Huang, Ying Zhu, Aihua Qian, Bin Xu and Weiyan Yao
Department of Gastroenterology. Ruijin Hospital. Shanghai Jiaotong University School of Medicine. Shanghai, China
Author's contribution: Yuming Tang and Jia Huang contributed equally to this work and should be considered co-first authors.
Financial support: This study was supported by the Medical Engineering Crossover Foundation of the Shanghai Jiao Tong University, China (Grant No.YG2014MS60).
ABSTRACT
Backgrounds and aims: This study aimed to compare the esophageal motility between gastroesophageal reflux disease (GERD) patients with typical symptoms but without globus sensation and GERD patients only with globus symptoms.
Methods: A total of 57 consecutive GERD patients diagnosed by endoscopy or by 24-hour pH monitoring between May 2013 and September 2015 were included retrospectively into the study. The patients were grouped based on the presence or absence of globus. Thirty patients presented with typical reflux symptoms but without globus were assigned to the typical GERD group and 27 patients only with globus symptom were assigned to the globus GERD group. All patients underwent esophageal high resolution manometry (HRM) and the differences in esophageal motility between the two groups were analyzed.
Results: The globus GERD group showed a significantly greater lower esophageal sphincter (LES) length, LES basal pressure and upper esophageal sphincter (UES) residual pressure than that of the typical GERD group (3.47 ± 0.76 vs 2.65 ± 0.62 cm, 21.71 ± 9.68 vs 16.04 ± 8.49 mmHg, 7.30 ± 4.42 vs 4.12 ± 2.92 mmHg, all p < 0.05). There was no significant difference between the two groups in terms of the distal wave amplitude, mean wave duration, distal contractile integral (DCI), contractile front velocity (CFV), distal latency (DL), integrated relaxation pressure (IRP) and UES basal pressure. The incidence of esophageal dysmotility in the globus GERD group (33.3%) was higher than in the typical GERD group (23.3%). There was no significant difference in esophageal acid exposure of the non-erosive gastroesophageal reflux disease (NERD) patients between the two groups.
Conclusions: Globus GERD patients have a higher UES residual pressure, longer LES length, higher LES basal pressure and greater esophageal dysmotility than typical GERD patients. HRM is useful in evaluating esophageal motility of GERD patients.
Key words: Esophageal high resolution manometry. Esophageal motility. Gastroesophageal reflux disease. Globus sensation.
Introduction
Globus sensation is a feeling of a lump or foreign body in the throat when no mass is actually there and this sensation is unrelated to dysphagia or odynophagia. The etiology of globus is not fully understood but seems to be multifactorial. The Rome III criteria include the "absence of evidence that gastroesophageal reflux is the cause of the symptom" as criteria to determine a diagnosis of globus (1). Recent studies suggest that GERD could be the major cause of globus, accounting for 23%-68% of patients with globus sensation (2). This study focused on the group of globus accompanied by GERD.
According to the Montreal definition, GERD is a condition which develops when the reflux of the stomach contents causes troublesome symptoms and/or complications. Heartburn and regurgitation are typical of the reflux syndrome (3). Besides, there are extra-esophageal symptoms including globus, chronic cough, hoarseness and asthma, among others. Previous studies have shown a close association between GERD and globus symptoms. A total of 38.7% of GERD patients had globus symptoms, which is even more frequent in those with non-erosive reflux disease (4). The proposed mechanisms of globus sensation in GERD include laryngopharyngeal irritation and inflammation caused by reflux (5) and vagovagal reflex hypertonicity of the UES caused by esophageal acidification or distention (6).
Esophageal dysmotility may also contribute to GERD. Previous studies have shown a higher frequency of esophageal dysmotility in GERD with globus rather than GERD with typical symptoms (7-9). High-resolution manometry (HRM) is a useful tool in evaluating esophageal motility (10) and this study aimed to compare the esophageal motility between GERD patients with and without globus symptoms. We speculated that different esophageal motility may contribute to the different symptoms.
Materials and methods
Subjects
A total of 57 consecutive GERD patients were retrospectively included in the study from May 2013 to September 2015. The diagnosis of GERD was based on symptoms as well as gastroscopy or 24-hour esophageal pH monitoring. The GERD patients enrolled in the study met the following inclusion criteria: a) typical symptoms (Heartburn and/or regurgitation) or the atypical symptom of globus sensation; b) a disease course longer than 0.5 years; c) undergone an esophageal high resolution manometry (HRM); d) undergone gastroscopy within 12 months before HRM; and e) reflux esophagitis (RE) detected by gastroscopy and for those with negative endoscopic findings, the reflux was confirmed by 24-hour esophageal pH monitoring. Patients with achalasia, diffuse esophageal spasm, nutcracker esophagus, pharyngeal organic diseases, coronary heart disease, cancer, peptic ulcer, esophageal varices and a history of digestive tract surgery were excluded. All patients signed an informed consent. The study was approved by the ethics committee of our hospital.
Thirty patients who presented with typical GERD symptoms (heartburn and/or regurgitation) but without globus sensation were assigned to the typical GERD group, while 27 patients with globus sensation but without typical symptoms were assigned to the globus GERD group. The disease course of GERD was 0.5-8 years. The BMI (body mass index) and consumption of tobacco and alcohol within the two groups were evaluated. Furthermore, the difference in esophageal motility between the two groups was compared.
Esophageal high resolution manometry
The Manoscan 360 system (Given Scientific Instruments Inc, Los Angeles, CA, USA) was adopted for esophageal high resolution manometry in our study. This system included a solid state manometry catheter with 36 circumferential pressure sensors spaced at 1 cm intervals and ManoView acquisition software to display pressure data. The equipment was calibrated for pressure and temperature according to the manufacturers' instructions before use.
After withdrawal of medication with known effects on gastrointestinal motility for at least 48 hours and fasting for at least 8 hours, the manometry catheter was placed through one nostril to the stomach with 3 intragastric sensors in an upright position. The pressure data were then acquired in a supine position after fixing the catheter to the nose.
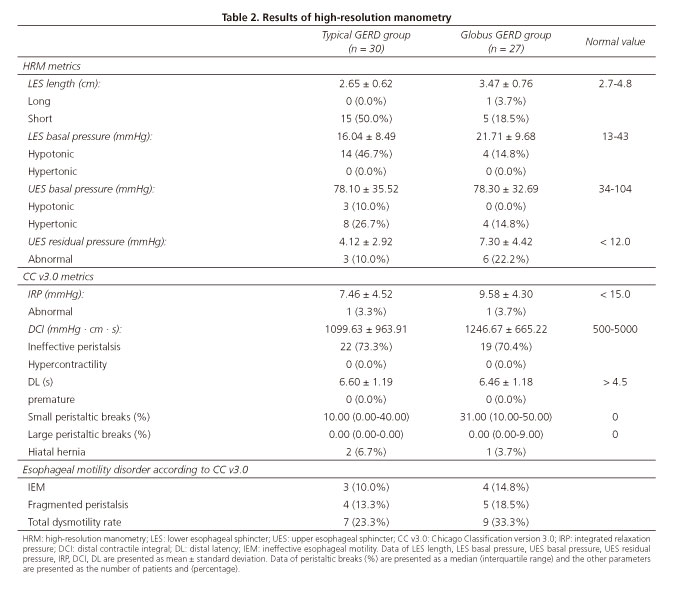
At least 30 seconds of basal sphincter pressure was recorded at the start, followed by ten successive 5 mL water swallows. The following HRM metrics were recorded including LES length, LES basal pressure, UES basal pressure, UES residual pressure, contractile front velocity (CFV), distal wave amplitude and mean wave duration. Moreover, the parameters included in the Chicago classification version 3.0 (11) such as integrated relaxation pressure (IRP), distal latency (DL) and distal contractile integral (DCI) were also evaluated. The esophageal motility disorders were diagnosed according to the Chicago classification version 3.0.
Mano ViewTM analysis software version 3.0 (Given Scientific Instruments Inc, Los Angeles, CA, USA) was used for data analysis. The normal ranges were as follows, LES length: 2.7-4.8 cm, LES basal pressure: 13-43 mmHg, IRP: < 15 mmHg, UES basal pressure: 34-104 mmHg, UES relaxation pressure: < 12 mmHg, DCI: 500-5000 mmHg · cm · sec (hypercontractile if DCI > 8000 mmHg · cm · sec and ineffective if DCI < 450 mmHg · sec · cm), CFV: < 9 cm/s, premature contraction was considered if DL was < 4.5 seconds, small peristaltic breaks was defined as 2-5 cm defects in the 20 mmHg isobaric contour and large peristaltic breaks was defined as defects > 5 cm in the 20 mmHg isobaric contour. Hiatal hernia was defined as a separation between the LES and crural diaphragm of > 2 cm (10).
Ambulatory 24-hour pH monitoring
Recording of the 24-hour esophageal pH monitoring was conducted with a multi-use VersaFlex® catheter (Given Scientific Instruments Inc, Los Angeles, CA, USA). The pH electrode was calibrated using pH 7.0 and pH 1.0 buffer solutions before the procedure. The catheter was transnasally placed and the electrode was positioned 5 cm above the proximal border of the LES. All patient data were recorded using the Digitrapper® equipment (Given Scientific Instruments Inc, Los Angeles, CA, USA). Abnormal esophageal acid exposure was defined as a total percentage time of greater than 4% with a pH < 4 and a DeMeester score > 14.7 (12).
Statistical methods
The SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. The Kolmogorov-Smirnov test was used to check the normal distribution of variables. Continuous data with a normal distribution were presented as mean ± standard deviation and those with a skewed distribution were presented as a median (25th, 75th percentile). The Student t test was used for the comparison of data with a normal distribution. The Mann-Whitney U test was used for non-parametric data. Differences were considered statistically significant when the p value was less than 0.05.
Results
Subjects demographics
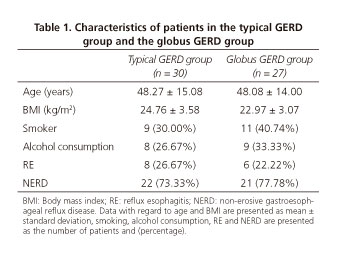
As shown in table 1, thirty typical GERD patients (17 males, 13 females) and 27 globus GERD (16 males, 11 females) patients were included in the study. The median age was 48.27 ± 15.08 (18-68) and 48.08 ± 14.00 (20-66) years, respectively. There was no difference in terms of gender and age between the two groups. The disease course of GERD was 0.5-8 years with a median of 3(2-4) [median (interquartile range)] years in the typical GERD group and 3.5(2.5-5) [median (interquartile range)] years in the globus GERD group (p = 0.523). Reflux esophagitis was detected by gastroscopy in 26.67% (8/30) of typical GERD patients and 22.22% (9/27) of globus GERD patients. The esophagitis cases were Los Angeles Grades A and B and no Barrett's esophagus were detected. Seventy percent (21/30) of typical GERD and 37.03% (10/27) of globus GERD patients received prior PPI treatment. Only 5 cases of typical GERD and 4 cases of globus GERD provided their gastroscopy reports before PPI use and these reports showed no severe esophagitis and Barrett's esophagus. There were no significant differences with regard to smoking, alcohol consumption and body mass index between the two groups.
High-resolution manometry parameters
Data with regard to esophageal motility is presented in table 2. Esophageal dysmotility accounted for 23.3% (7/30) of typical GERD patients and 33.3% (9/27) of globus GERD patients based on the Chicago classification 3.0. All were minor motility disorders including ineffective esophageal motility (IEM) and fragmented peristalsis. A comparison of HRM findings between the two groups are described in table 3. A greater LES length (3.47 ± 0.76 vs 2.65 ± 0.62 cm), LES basal pressure (21.71 ± 9.68 vs 16.04 ± 8.49 mmHg) and UES residual pressure (7.30 ± 4.42 vs 4.12 ± 2.92 mmHg) were found in the globus GERD group. However, no difference was found with regard to distal wave amplitude, mean wave duration, IRP, DCI, DL, CFV and UES basal pressure (Table 3 and Fig. 1). There were significantly more small peristaltic breaks in the globus GERD group than the typical GERD group (31% vs 10%, p = 0.035). There was no difference with regard to large peristaltic breaks between the two groups (p = 0.459). Hiatal hernias were found in 6.7% (2/30) of the patients in the typical GERD group and 3.7% (1/27) of patients in the global GERD group.
Ambulatory 24-hour pH monitoring
In our retrospective study, 43 NERD patients with negative endoscopic findings were diagnosed by ambulatory 24-hour pH monitoring. Among these NERD patients, there were 22 typical GERD and 21 globus GERD. The difference in the total fraction of time with a pH < 4 between the typical GERD group and the globus GERD group was not significant [7.1% (5.1-14.8) vs 6.9% (4.7-12.9), p = 0.625]. This was also true for the DeMeester score [26.3 (16.7-41.4) vs 24.5 (15.2-37.2), p = 0.343].
Discussion
GERD patients may have various extraesophageal symptoms (EES) such as chronic cough, asthma, laryngitis and globus, among others. In Korea, the prevalence of EES was about 90.3% in GERD and globus sensation was the second most common EES (51.8%) (13,14). Globus sensation may appear with or without typical GERD symptoms. Hori et al. (15) observed that globus sensation was strongly associated with non-erosive reflux disease rather than reflux esophagitis. Likewise, we found that 77.78% of the globus GERD group had NERD and less had RE. However, there were no differences in the prevalence of NERD and RE between the globus GERD group and the typical GERD group. We found no severe esophagitis in the current study, which may be partially explained by previous PPI treatments. Seventy percent of the patients with typical GERD and 37.03% with globus GERD received prior PPI treatment. Only 5 patients with typical GERD and 4 patients with globus GERD provided the gastroscopy reports before PPI use, there were no cases of severe esophagitis and Barrett's esophagus. In addition, most of the reflux esophagitis were of a mild grade (LA A, B) and less cases were severe esophagitis (LA C, D) (16,17) within the Chinese population. The prevalence of Barrett's esophagus was generally low in Asian patients, ranging from 0.9% to 2% (18).
Gastroesophageal reflux has been suggested to be a major cause of globus sensation, accounting for 23%-68% of globus patients (2). Chevalier et al. (19) found significant episodes of acid reflux in 66.6% of globus patients without typical gastroesophageal reflux (GER) symptoms and 80% of those with typical GER symptoms. Nowadays, multichannel intraluminal impedance and pH monitoring (MII-pH) have become important tools to detect reflux. Data from MII-pH suggested that globus may be due to non-acid (NAR) reflux (20). However, it remains unclear whether there is a difference between globus GERD and typical GERD with regard to GER. In our study, the globus GERD group had a longer LES length and higher LES basal pressure than the typical GERD group, whereas the acid reflux was the same. The esophageal acidification induced vagovagal reflex hypertonicity that probably contributed to the globus sensation in these patients. One limitation of the current study is that we used ambulatory 24-hour pH monitoring and the interference of non-acid reflux events was not excluded.
Esophageal motor disorders have been demonstrated in patients with typical GER and served as a possible cause of globus sensation. Knight et al. (7) found esophageal dysmotility in 75% of GERD patients with globus symptoms. For those who were resistant to PPI therapy, Tsutsui et al. (8) reported a prevalence of abnormal esophageal motility of 47.9%. However, in the study by Ribolsi et al. (9), only 24% of patients with typical GERD symptoms demonstrated esophageal dysmotility. In our study, the prevalence of esophageal dysmotility in the globus GERD group was 33.3% and 23.3% in the typical GERD group. The different rate of esophageal dysmotility may be due to the different enrollment criteria of globus patients. We included GERD patients with a sole globus sensation who lacked typical symptoms. The study by Knight et al. included those with mixed symptoms and the study by Tsutsui et al. focused on those with globus sensation and a poor response to PPI therapy.
Whether UES hypertonicity contributes to globus is controversial. An earlier study revealed that a respiration-related change in resting UES pressure was significantly amplified in globus patients (21). However, Choi et al. (22) evaluated patients with globus, gastroesophageal reflux disease and normal controls by high-resolution manometry and found no significant difference in the UES pressure. Sun et al. (23) found that UES pressure was normal in most of the globus patients, while pharyngeal dysfunction had a strong association with globus. Wilson et al. (24) noticed that globus patients exhibited higher pharyngeal and upper esophageal sphincter after-contraction pressures during deglutition. Recently, Peng et al. found an elevated UES residual pressure in globus patients (25). In the current study, we found that the globus GERD group had a significantly higher UES residual pressure than the typical GERD group, while no difference in UES basal pressure was found between the two groups. These results were supportive of the role of elevated UES residual pressure in the pathogenesis of globus. Visceral hypersensitivity and central hypervigilance was the potential pathophysiological mechanism (26). Esophageal high resolution manometry can provide information of esophageal motility in GERD with typical and atypical symptoms which may aid in a more profound study of GERD.
There were limitations in our study. The results may be limited by the small sample size, there were both NERD and RE cases in the study groups and the heterogeneity may lead to a bias. It was difficult to enroll globus patients without typical GERD symptoms as most of them would go to the ear, nose and throat (ENT) clinic first for globus treatment and very few cases visit the gastrointestinal (GI) clinic. In the future, large prospective controlled studies are needed to determine the difference between the globus GERD and typical GERD groups.
References
1. Galmiche JP, et al. Functional esophageal disorders. In: Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III. The functional gastrointestinal disorders. 3rd. Degnon Associates, Inc; 2006. pp. 396-418. [ Links ]
2. Lee BE, Kim GH. Globus pharyngeus: a review of its etiology, diagnosis and treatment. World J Gastroenterol 2012;18:2462-71. DOI: 10.3748/wjg.v18.i20.2462. [ Links ]
3. Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20;quiz 1943. DOI: 10.1111/j.1572-0241.2006.00630.x. [ Links ]
4. Dore MP, Pedroni A, Pes GM, et al. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig Dis Sci 2007;52(2):463-8. DOI: 10.1007/s10620-006-9573-7. [ Links ]
5. Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005;294:1534-40. DOI: 10.1001/jama.294.12.1534. [ Links ]
6. Tokashiki R, Funato N, Suzuki M. Globus sensation and increased upper esophageal sphincter pressure with distal esophageal acid perfusion. Eur Arch Otorhinolaryngol 2010;267:737-41. DOI: 10.1007/s00405-009-1134-1. [ Links ]
7. Knight RE, Wells JR, Parrish RS. Esophageal dysmotility as an important co-factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope 2000;110:1462-6. DOI: 10.1097/00005537-200009000-00010. [ Links ]
8. Tsutsui H, Manabe N, Uno M, et al. Esophageal motor dysfunction plays a key role in GERD with globus sensation--analysis of factors promoting resistance to PPI therapy. Scand J Gastroenterol 2012;47:893-9. DOI: 10.3109/00365521.2012.685756. [ Links ]
9. Ribolsi M, Balestrieri P, Biasutto D, et al. Role of Mixed Reflux and Hypomotility with Delayed Reflux Clearance in Patients with Non-cardiac Chest Pain. J Neurogastroenterol Motil 2016;22(4):606-12. DOI: 10.5056/jnm15182. [ Links ]
10. Ruiz de León A, Ciriza C, Pérez de la Serna J, et al. Practical aspects of high resolution esophageal manometry. Rev Esp Enferm Dig 2017;109(2):91-105. [ Links ]
11. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0.Neurogastroenterol Motil 2015;27(2):160-74. DOI: 10.1111/nmo.12477. [ Links ]
12. Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992;87(9):1102-11. [ Links ]
13. Saritas Yuksel E, Vaezi MF. New developments in extraesophageal reflux disease. Gastroenterol Hepatol (NY) 2012;8:590-9. [ Links ]
14. Min YW, Lim SW, Lee JH, et al. Prevalence of Extraesophageal Symptoms in Patients With Gastroesophageal Reflux Disease: A Multicenter Questionnaire-based Study in Korea. J Neurogastroenterol Motil 2014;20(1):87-93. DOI: 10.5056/jnm.2014.20.1.87. [ Links ]
15. Hori K, Kim Y, Sakurai J, et al. Non-erosive reflux disease rather than cervical inlet patch involves globus. J Gastroenterol 2010;45(11):1138-45. DOI: 10.1007/s00535-010-0275-8. [ Links ]
16. Chen MJ, Lee YC, Chiu HM, et al. Time trends of endoscopic and pathological diagnoses related to gastroesophageal reflux disease in a Chinese population: eight years single institution experience. Dis Esophagus 2010;23(3):201-7. DOI: 10.1111/j.1442-2050. 2009.01012.x. [ Links ]
17. Chen TS, Chang FY. The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan. J Clin Gastroenterol 2007;41(9):819-22. DOI: 10.1097/01.mcg.0000225658.30803.79. [ Links ]
18. Fock KM, Talley NJ, Fass R, et al. Asia-Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol. 2008;23(1):8-22. DOI: 10.1111/j.1440-1746.2007.05249.x. [ Links ]
19. Chevalier JM, Brossard E, Monnier P. Globus sensation and gastroesophageal reflux. Eur Arch Otorhinolaryngol 2003;260(5):273-6. [ Links ]
20. Anandasabapathy S, Jaffin BW. Multichannel intraluminal impedance in the evaluation of patients with persistent globus on proton pump inhibitor therapy. Ann Otol Rhinol Laryngol 2006;115(8):563-70. DOI: 10.1177/000348940611500801. [ Links ]
21. Kwiatek MA, Mirza F, Kahrilas PJ, et al. Hyperdynamic upper esophageal sphincter pressure: a manometric observation in patients reporting globus sensation. Am J Gastroenterol 2009;104:289-98. DOI: 10.1038/ajg.2008.150. [ Links ]
22. Choi WS, Kim TW, Kim JH, et al. High-resolution Manometry and Globus: Comparison of Globus, Gastroesophageal Reflux Disease and Normal Controls Using High-resolution Manometry. J Neurogastroenterol Motil 2013;19:473-8. DOI: 10.5056/jnm.2013.19.4.473. [ Links ]
23. Sun J, Xu B, Yuan Y, et al. Study on the function of pharynx & upper esophageal sphincter in globus hystericus. World J Gastroenterol 2002;8(5):952-5. DOI: 10.3748/wjg.v8.i5.952. [ Links ]
24. Wilson JA, Pryde A, Piris J, et al. Pharyngoesophageal dysmotility in globus sensation. Arch Otolaryngol Head Neck Surg 1989;115(9):1086-90. DOI: 10.1001/archotol.1989.01860330076021. [ Links ]
25. Peng L, Patel A, Kushnir V, et al. Assessment of upper esophageal sphincter function on high-resolution manometry: identification of predictors of globus symptoms. J Clin Gastroenterol 2015;49(2):95-100. DOI: 10.1097/MCG.0000000000000078. [ Links ]
26. Chen CL, Szczesniak MM, Cook IJ. Evidence for oesophageal visceral hypersensitivity and aberrant symptom referral in patients with globus. Neurogastroenterol Motil 2009;21(11):1142-e96. DOI: 10.1111/j.1365-2982.2009.01316.x. [ Links ]
![]() Correspondence:
Correspondence:
Weiyan Yao.
Department of Gastroenterology.
Ruijin Hospital.
Shanghai Jiaotong University School of Medicine.
No. 197, Ruijin 2nd Road.
Shanghai 200025, China
e-mail: yaowymail@sina.com
Received: 18-05-2017
Accepted: 29-07-2017