Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.40 no.1 Toledo ene./feb. 2016
https://dx.doi.org/10.7399/fh.2016.40.1.9339
ORIGINALES
Effect of a single dose of lidocaine and ketamine on intraoperative opioids requirements in patients undergoing elective gynecological laparotomies under general anesthesia. A randomized, placebo controlled pilot study
Efecto de una dosis de lidocaína y ketamina sobre el consumo intraoperatorio de opioides en pacientes sometidas a cirugía ginecológica electiva bajo anestesia general. Estudio piloto aleatorizado y controlado con placebo
Jusset Teresa García-Navia1, Javier Tornero López1, Juan José Egea-Guerrero2, Ángel Vilches Arenas3 and Tiburcio Vázquez Gutiérrez1
1Hospital Universitario Nuestra Señora de Valme, Universidad de Sevilla, Sevilla.
2Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío, CSIC, Universidad de Sevilla, Unidad de Neurocríticos, Sevilla.
3Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío, CSIC, Universidad de Sevilla, Departamento de Medicina Preventiva y Salud Pública, Sevilla. Spain.
This study was supported by institutional and departmental resources.
ABSTRACT
Background and goal of study: there is evidence that perioperative intravenous ketamine and lidocaine reduce postoperative pain, postoperative opioids consumption, shortens hospital stay and accelerates intestinal function recovery. However, it has not been studied the beneficial effects in the intraoperative period. The aim of this study was to evaluate the effect of a single dose of lidocaine and ketamine on intraoperative opioids requirements in patients undergoing elective gynecological laparotomies under general anesthesia.
Materials and methods: we performed a single-centre, prospective, randomized, double-blinded, placebo-controlled study. We included 33 patients (11 in the ketamine group, 11 in the lidocaine group and 11 in the placebo group). Postoperative analgesia was accomplished by patient-controlled morphine. Patients were randomly assigned to receive either a 1.5 mg/kg of 2% lidocaine, 0.5 mg/kg of 5% ketamine or 0.9% saline bolus. The primary outcome was the opioids consumption during surgery. The secondary outcomes included: emergence time, pain scores, opioids consumption within 24 h after surgery and side effects.
Results: decreased intraoperative opioids requirements were noted in the experimental groups (ketamine: 402.3 ± 106.3 and lidocaine: 397.7 ± 107.5, compared with saline: 561.4 ± 97.1); p = 0.001. We found a positive correlation between intraoperative opioids consumption and emergence time (r = 0.864, p < 0.001). There was no significant difference between the groups in VAS pain scores at rest within the first 24 postoperative hours. Total morphine consumption within 24 h after surgery did not differ significantly among the groups (placebo: 27.54 ± 11.75; ketamine: 30.95 ± 7.88; lidocaine 34.77 ± 10.25; p = 0.26). Postoperative nausea and vomiting were more common in placebo group (it was observed in 3 subjects in ketamine group, in 5 subjects in lidocaine group and in 9 subjects in placebo group; p = 0.027).
Conclusion: our results do not support the use of intraoperative single dose of lidocaine or ketamine to reduce postoperative pain and postoperative opioids consumption after open gynecological surgery. However, they seem to decrease intraoperative opioids requirements and shorten emergence time. Nevertheless, these findings should be validating in further studies with large sample size.
Key words: Ketamine; Lidocaine; Analgesic agents; Perioperative period; Laparotomy.
RESUMEN
Introducción y objetivos del estudio: existe evidencia de que la administración perioperatoria de ketamina y lidocaína intravenosa reduce el dolor y el consumo de opioides postoperatorio, acorta la estancia hospitalaria y acelera la recuperación de la función intestinal. Sin embargo, no se han estudiado los efectos beneficiosos en el período intraoperatorio. El objetivo de este estudio fue evaluar el efecto de una única dosis de lidocaína y ketamina sobre el consumo intraoperatorio de opioides en pacientes sometidas a cirugía ginecológica electiva bajo anestesia general.
Material y métodos: estudio prospectivo, aleatorizado, doble ciego, controlado con placebo en un solo centro. Se incluyeron 33 pacientes (11 en el grupo ketamina, 11 en el grupo lidocaína y 11 en el grupo placebo). Para la analgesia postoperatoria se utilizó una bomba PCA (Analgesia Controlada por el Paciente ) de morfina. Los pacientes fueron asignados al azar a uno de los tres grupos de estudio: 1,5 mg/kg de lidocaína al 2%, 0,5 mg/kg de ketamina al 5% o solución salina 0.9%. La variable principal del estudio fue el consumo de opioides durante la cirugía. Las variables secundarias fueron: tiempo de educción de la anestesia, intensidad del dolor, consumo de opioides en las 24 horas posteriores a la cirugía y efectos adversos.
Resultados: se observó una disminución del consumo intraoperatorio de opioides en los grupos ketamina (402,3 ± 106,3) y lidocaína (397,7 ± 107,5) frente al grupo placebo (561,4 ± 97,1); p = 0,001. Se encontró una correlación positiva entre el consumo intraoperatorio de opioides y el tiempo de despertar (r = 0,864, p <0,001). No hubo diferencias significativas respecto a la intensidad del dolor en reposo en las 24 horas posteriores a la cirugía. El consumo total de morfina en las primeras 24 horas tras la cirugía no difirió significativamente entre los grupos (placebo: 27,54 ± 11,75; ketamina: 30,95 ± 7,88; lidocaína 34,77 ± 10,25; p = 0,26). Las náuseas y vómitos postoperatorios fueron más frecuentes en el grupo placebo (se observó en 3 pacientes del grupo ketamina, en 5 del grupo lidocaína y en 9 del grupo placebo; p = 0,027).
Conclusión: Nuestros resultados no apoyan el uso de una única dosis intraoperatoria de lidocaína o ketamina para disminuir el dolor postoperatorio y el consumo de opioides tras cirugía ginecológica abierta. Sin embargo, si parece disminuir los requerimientos intraoperatorios de opioides y acortar el tiempo de educción de la anestesia. No obstante, estos resultados deben ser validados en futuros estudios con mayor tamaño muestral.
Palabras clave: Ketamina; Lidocaína; Fármacos analgésicos; Período perioperatorio; Laparotomía.
Introduction
Opioids are widely used in the perioperative period because of their profound analgesic effect. However, this drug can be associated with undesirable side effects, which may include respiratory depression, hypotension, sedation, nausea and vomiting, urinary retention and postoperative ileus1-3. Side effects related with analgesia in surgical patients can be problematic and can lead to increased institutional costs, longer hospital length of stay, and an overall increase in patient dissatisfaction4-6.
Given the multiplicity of mechanisms involved in pain pathophysiology, an appropriate approach would be a multimodal analgesia regimen that uses a combination of opioids and multiple agents aiming at augmenting their reciprocal effects7-9.
One medication that has shown promise in providing analgesia effect is lidocaine. This is an amide local anesthetic agent that works by blocking sodium channels in the neural cascade. Recent investigations suggest that intravenous lidocaine may be beneficial in biochemical pain processes4,6,10-12.
Although the exact mechanism and analgesic-sparing effect of parenterally administered lidocaine is unclear, some authors speculate that it acts more as an antihyperalgesic than as a direct analgesic. Is known central sensitization to be induced by the mechanosensitive nociceptor class of receptors. These nociceptors are known to be sensitive to small-dose lidocaine10.
Moreover, important mechanisms in the genesis of pain include N-methyl-D-aspartate receptor (NMDAR) activation and up-regulation and inflammatory responses in the spinal cord13-15. In this regard, ketamine, a non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, has received increased interest in recent years as an analgesic for acute pain management. At relatively high dose, ketamine produces anesthesia, whereas at sub-anesthetic dose, it is a potent analgesic. Low doses of ketamine have been proposed to prevent opioid tolerance and opioid-induced-hyperalgesia16,17.
The effect of ketamine on perioperative inflammatory responses has been studied in patients undergoing total hip arthroplasty, hysterectomy, thoracotomy, cardiac and spine surgery18-22.
Thus, these agents could have an important analgesic effect not only in the postoperative period, but also in the intraoperative period. Therefore, the main objective of this study was to evaluate the effect of a single dose of lidocaine and ketamine on intraoperative opioids requirements in patients undergoing elective gynecological laparotomies under general anesthesia.
Materials and Methods
This was a single-centre, prospective, randomized, double-blinded, placebo-controlled design. The patients were recruited for participation in the study after approval of the protocol by the local medical ethics committee of Nuestra Señora de Valme University Hospital, Seville, Spain. Informed written consent was obtained from all participants according to the Declaration of Helsinki.
Thirty-three female, aged 18-55 years, undergoing elective open gynecological surgery under general anesthesia were enrolled in the study.
Exclusion criteria were: age <18 years or >55 years, preexisting chronic pain requiring treatment, contraindication to ketamine or lidocaine, oncologic surgery, history of significant neurological or psychiatric disease (e.g., increased cranial pressure, seizure disorder requiring medication within the previous 2 years, psychosis), substantial hepatic (alanine aminotransferase or aspartate aminotransferase >2 times normal) or renal impairment (serum creatinine >2 mg/dL) and previous substance abuse.
The primary outcome was the opioids consumption during surgery. The secondary outcomes were the emergence time, pain scores, opioids consumption within 24 h after surgery and side effects.
Patients were randomly assigned, using computer-generated random numbers and concealed opaque envelopes to receive either, a 1.5 mg/kg of 2% lidocaine, 0,5 mg/kg of 5% ketamine or 0.9% saline bolus.
The day of surgery, coded 10-mL syringes of lidocaine, ketamine, or saline were prepared by the nurse in the Post-Anesthesia Care Unit (PACU). Each syringe was indistinguishable from the other, and only the nurse who prepared the syringes was aware of their actual composition. The anesthesiologist in charge, the surgeon, the nursing staff and the patients were blind to the group assignment until the conclusion of the study.
Every patient was admitted one day before surgery according to institutional standards. In the operating room, intravenous access was secured, and routine monitoring was established using an electrocardiogram, non-invasive blood pressure, heart rate, oxygen saturation and end-tidal carbon dioxide (Et CO2) (Datex-Ohmeda S/5 Compact Critical Care Monitor; GE Healthcare). All patients were preoxygenated with 100% oxygen via facemask for 3 to 5 minutes before induction of anesthesia. Anesthesia was induced with an intravenous administration of 1-2 mg of midazolam, 2 μg/kg of 0.005% fentanyl and 2 mg/kg of 1% propofol. Orotracheal intubation was facilitated with 0,6 mg/kg of 1% rocuronium and respiratory frequency and tidal volume were adjusted to maintain the Et CO2 between 32 to 35 mmHg. Dexamethasone 4 mg i.v could be administered during induction of anesthesia in all patients with moderate or high risk of postoperative nausea and vomiting (PONV), according Apfel's model23,24.
Maintenance of anesthesia was accomplished using propofol according to the Bispectral Index (BIS) and additional fentanyl was administered as needed during anesthesia with a 20% increase in blood pressure or if the heart rate was greater than 100 beats/min. The total amount of fentanyl used were recorded at the end of the procedure. Rocuronium boluses were given for intraoperative muscle relaxation to maintain a moderate neuromuscular block to train-of-four stimulation (TOF).
The anesthetist administered lidocaine, ketamine, or saline bolus 5 minutes before the surgical incision.
Aproximately 15 to 30 minutes before skin closure 4 mg of 0,2% ondansetron, 2g of 40% metamizol and 50 mg of 2,5% dexketoprofen were administered in all groups. Neuromuscular blockade was antagonized using IV neostigmine, 0.05 mg/kg, and atropine 0.015 mg/kg after the reappearance of T4 in the TOF.
Emergence time from anesthesia was collected (measured in minutes from discontinuation of propofol infusions to the moment at which the patient awoke and endotracheal tube was removed).
After removal of the endotracheal tube, subjects were transported to the PACU. Total surgical time (measured in minutes from surgical incision to skin closure) was recorded.
On admission to the PACU, a postoperative pain was assessed as soon as the subject was alert and able to answer questions. A level of abdominal pain was assessed using a 0 to 10 Verbal Analogue Scale (VAS), in which a rating of "0" indicated "absence of pain" and a score of "10" indicated the "worst pain imaginable". VAS also was recorded at 2, 4, 8 and 24 h postoperatively.
At the same time points, sedation (was scored as 1 = alert, 2 = asleep, alert after arousal, 3 = asleep, drowsy after arousal, 4 = asleep, difficult to rouse, and 5 = unarousable), hallucinations, pruritus, incidence of any nausea or vomiting, ileus, respiratory depression and any signs and symptoms of unexpected or major side effects were assessed. Research personnel, blind to the allocation, recorded these variables.
The amount of intraoperative opioid use was recorded. Patients were connected to morphine-PCA and morphine was given intravenously during the first 24 h in boluses of 1,5 mg using Patient Controlled Analgesia (PCA), with a minimum interval of 10 min between two doses. No other analgesics were used. The analgesic requirements for the first 24 hours were recorded.
Statistical Analysis
The distribution of data was determined using the Kolmogorov-Smirnov analysis.
Data were expressed as mean (standard deviations), and nonparametric data as median (interquartile range). Categorical data were compared among groups using chi-square test and Fisher exact test. Parametric data were compared using an unpaired student's t-test, and nonparametric data using a Mann-Whitney test. To compare numerical variables between more than two groups ANOVA was conducted. Data that were not normally distributed were compared among groups using a Kruskal-Wallis test. The relationship between numerical variables was calculated using the Pearson correlation coefficient. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using software from the Statistical Package for the Social Sciences (SPSS, Version 18.0, Chicago, IL, USA).
Results
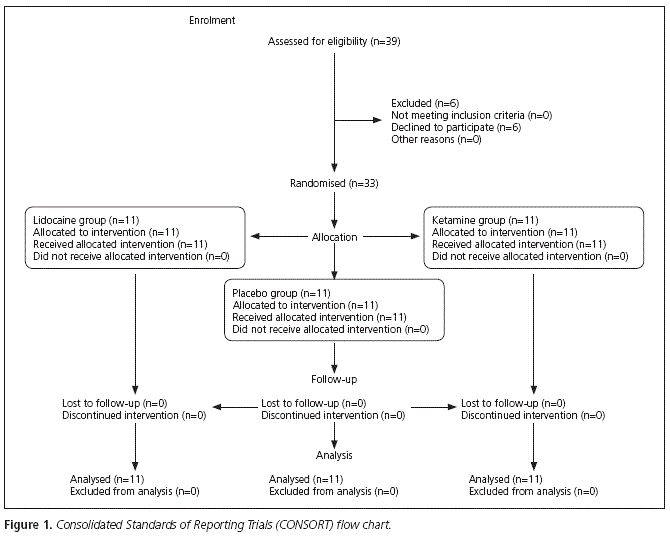
Thirty-three patients were analyzed (11 in the ketamine group, 11 in the lidocaine group and 11 in the placebo group). All subjects completed the protocol without unexpected or major side effects. Patient flow throughout the study, according to the CONSORT statement, is shown in figure 1.
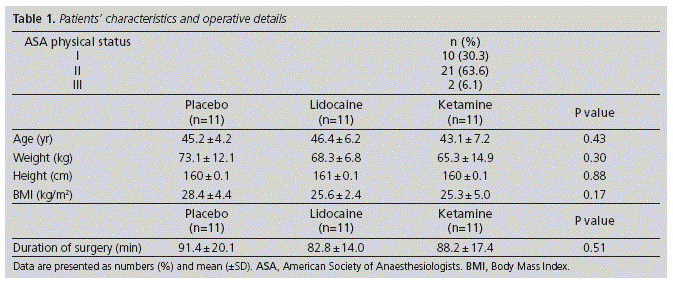
There were no differences in age, weight, height, body mass index or duration of surgery between treatment groups (Table 1).
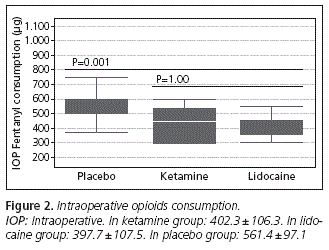
Decreased intraoperative fentanyl requirements were noted in the experimental groups compared with the control group (Figure 2). However, the ketamine group and lidocaine group did not differ significantly among them (p = 1.00).
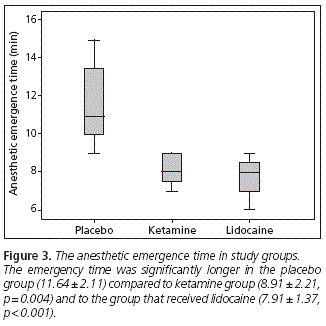
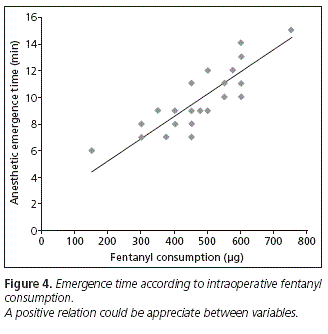
The emergency time was significantly longer in the placebo group (11.64 ± 2.11) compared to ketamine group (8.91 ± 2.21, p = 0.004) and to the group that received lidocaine (7.91 ± 1.37, p < 0.001). However, there were no significant differences in emergency time between ketamine and lidocaine groups (p = 0.270) (Figure 3). As shown in figure 4, we found a positive correlation between intraoperative fentanyl consumption and emergence time (r = 0.864, p < 0.001). The highest intraoperative fentanyl requirements observed in the placebo group compared to other groups, was probably a condition that may have contributed to an increase in emergency time in this group.
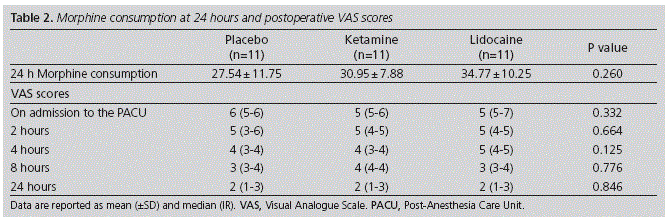
Morphine PCA was used for 24 h by 11 patients in each group. Table 2 shows the morphine consumption for groups during the first 24 postoperative hours. The mean ± SD total morphine consumption did not differ significantly among groups (placebo groups 27.54 ± 11.75, ketamine group 30.95 ± 7.88 and lidocaine group 34.77 ± 10.25; p = 0.260). There was no difference between the groups in VAS pain scores at rest from 0 to 24 hours postoperatively. A trend toward lower VAS scores was noted at 24 hours after surgery in all groups (Table 2).
No patients complained of hallucinations, dysphoria or disorientation. Sedation scores were similar among groups, and no patient had a score more than 2. Statistically significant difference in the incidence of PONV was found between groups. PONV was observed in 3 subjects in ketamine group, in 5 subjects in lidocaine group and in 9 subjects in placebo group (p = 0.027). Regarding itching, there was no significant difference among groups (p > 0.05). No other adverse events were observed in the postoperative period. There were no surgical complications.
Discussion
This study shows 2 main findings. First, a small intravenous single dose of lidocaine and ketamine given before skin incision reduces intraoperative opioids requirements in patients undergoing gynecological laparotomies under general anesthesia. Second, the subjects in the lidocaine and ketamine groups had substantially shorter emergence time than the placebo group. These findings are particularly important in the ambulatory surgery population because these patients do not have the structured support of a hospital staff and are, therefore, expected to have a faster recovery.
In our study, no intravenous infusion of lidocaine and ketamine was use, an aspect that differentiates this study from most previous researches. The lack of significant differences between the groups in VAS pain scores and opioids consumption from 0 to 24 hours postoperatively may be influenced by this fact.
Lidocaine regimens vary amongst the studies included in our study. Most of the studies have reported lower pain scores and decreased postoperative opioids requirement used intravenous infusion of lidocaine after bolus dosing25-30. The half-life of lidocaine has been reported to be about 100 minutes following bolus injection or infusions lasting less than 12 hours, showing more linear pharmacokinetics31. Therefore, after a single dose of lidocaine the dip in concentrations to below the therapeutic range occurs 1-3 hours after its administration32.
When systemic lidocaine is administered in the operative period, it likely prevents the induction of central hyperalgesia10. Grigoras A et al.33, who observed a decrease the incidence of persistent pain after breast surgery, highlight this fact.
Systematic review of multiple studies documented a reduction in perioperative pain when IV lidocaine infusions supplement general anesthetics4. However, there are also exceptions, Wuethrich PY et al.34 found that systemic perioperative lidocaine administration over 24 h did not influence opioid consumption after laparoscopic renal surgery.
The acute analgesic effects of ketamine are generally believed to be mediated through the blockade of phencyclidine binding site of N-methyl-d- aspartate (NMDA) receptors of the nociceptive neurons. In addition, various studies, including clinical and preclinical research, have shown that ketamine has an anti-inflammatory effect35,36. However, is currently unclear if the anti-inflammatory effect it is mediated by NMDA or non-NM-DA mechanisms.
One review concluded that a single bolus dose of ketamine decreased IV and epidural opioids requirements in seven of 11 studies37. A more recent Cochrane review analyzed 37 studies (2240 patients) and found that subanesthetic ketamine doses were effective in reducing 24 h morphine requirements and the incidence of PONV; the adverse effects were mild or absent38. Our results showed a lower incidence of PONV in the ketamine group, but there was no difference in opioid consumption at 24 hours postoperatively.
Ketamine combined with opioids potentiates analgesia, thus reducing the need for large opioids doses39,40. It has been estimated that pain is reduced by 20-45%39,41,42. Nesher N et al.42 have demonstrated that even smaller IV doses (≤ 250 mcg/kg) effectively controlled opioid-resistant pain, although it was associated with a brief period of sedation (< 2 min) immediately after the IV injection, but with no psychomimetic events.
The data of ketamine use available in the literature has led to conclude that it can reduce postoperative pain intensity, based on Level A evidence. The incidence of its benefit in reducing postoperative nausea and vomiting (PONV) has been confirmed by Level B evidence, and the effect on lowering the required dose of morphine by 3050% by Level A evidence43. Regarding adverse events, ketamine increased the incidence of neuropsychiatric effects (eg, hallucinations) and decreased the incidence of postoperative nausea and vomiting, but did not alter the incidence of sedation or other side effects16.
The limitations of this study include reliance on the accuracy of patient self-reported data and reliance on a convenience sample recruited from 1 centre, which may limit the generalisability of the results. Our study was restricted to women by nature of the surgery. Regarding adverse events, we consider a larger sample of patients would be needed to draw conclusions. Finally, although the monitoring of neuromuscular blockade was performed in all patients, the train-of-four was not collected, a point to be considered in future studies.
In summary, our results do not support the use of intraoperative single dose of lidocaine or ketamine to reduce postoperative pain and postoperative opioids consumption after open gynecological surgery. However, they seem to decrease intraoperative opioids requirements and shorten emergence time, particularly important in the ambulatory surgery population. Nevertheless, these findings should be validating in further studies with large sample size.
Conflict of interest
None declared.
Bibliography
1. White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg 2005;101(5 Suppl):S5-22. [ Links ]
2. Brown AK, Christo PJ, Wu CL. Strategies for postoperative pain management. Best Pract Res Clin Anaesthesiol 2004;18:703-17. [ Links ]
3. Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America 2005;23:185-202. [ Links ]
4. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010;70:1149-63. [ Links ]
5. Herroeder S, Pecher S, Schönherr ME, Kaulitz G, Hahnenkamp K, Friess H, Böttiger BW, Bauer H, Dijkgraaf MG, Durieux ME, Hollmann MW. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246:192-200. [ Links ]
6. Groudine SB, Fisher HAG, Kaufman RP Jr, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235-9. [ Links ]
7. Carstensen M, Moller AM. Adding ketamine to morphine for intravenous patient controlled analgesia for acute postoperative pain: a qualitative review of randomized trials. Br J Anaesth 2010;104:401-6. [ Links ]
8. Bonnet F, Marret E. Postoperative pain management and outcome after surgery. Best Pract Res Clin Anaesthesiol 2007;21:99-107. [ Links ]
9. Chelly JE, Ploskanych T, Dai F, Nelson JB. Multimodal analgesic approach incorporating paravertebral blocks for open radical retropubic prostatectomy: a randomized double-blind placebo-controlled study. Can J Anaesth 2011;58:371-8. [ Links ]
10. Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, Hering W. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 2004;98:1050-1055. [ Links ]
11. Farag E, Ghobrial M, Sessier DI, Dalton JE, Liu J, Lee JH, Zaky S, Benzel E, Bingaman W, Kurz A. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 2013;119:932-40. [ Links ]
12. Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain 2012;28:567-72. [ Links ]
13. Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg 2003;97:1108-16. [ Links ]
14. Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci 2009;30:229-41. [ Links ]
15. Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines pain enhancement. Neurosignals 2005;14:166-74. [ Links ]
16. Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-d-aspartate-dependant pain facilitatory systems. Neuroscience 1998;84:583-9. [ Links ]
17. Kissin I, Bright CA, Bradley EL Jr. The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg 2000;91:1483-8. [ Links ]
18. Martínez V, Cymerman A, Ben Ammar, Flaud JF, Rapon C, Poindessous F, Judet T, Chauvin M, Bouhassira D, Sessier D, Mazoit X, Fletcher D. The analgesic efficiency of combined pregabalin and ketamine for total hip arthroplasty: a randomised, double-blind, controlled study. Anaesthesia 2014;69:46-52. [ Links ]
19. Grady MV, Mascha E, Sessier DI, Kurz A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesth Analg. 2012 Nov;115(5):1078-84. [ Links ]
20. Mendola C, Cammarota G, Netto R, Cecci G, Pisterna A, Ferante D, Casadio C, Della Corte F. S+ -ketamine for control of perioperative pain and prevention of post thoracotomy pain syndrome: a randomized, double-blind study. Minerva Anestesiol. 2012;78:757-66. [ Links ]
21. Welters ID, Feurer MK, Preiss V, Müller M, Scholz S, Kwapisz M, Mogk M, Neuhäuser C. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br J Anaesth. 2011;106:172-9. [ Links ]
22. Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639-46. [ Links ]
23. Apfel CC, Roewer N, Kortila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand 2002;46:921-28. [ Links ]
24. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693-700. [ Links ]
25. Kuo CP, JaoSW, Chen KM, Wong CS, Yeh CC, Sheen MJ, Wu CT. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth 2006;97:640-6. [ Links ]
26. Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessier H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg 2009;109:1464-9. [ Links ]
27. Kaba A, Laurent SR, Detroz BJ, Sessier DI, Durieux ME, Lamy ML, Joris JL. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 2007;106:11-8. [ Links ]
28. Lauwick S, Kim do J, Michelagnoli G, Mistraletti G, Feldman L, Fried G, Carli F. Intraoperative infusion of lidocaine reduces postoperative fentanyl requirements in patients undergoing laparoscopic cholecystectomy. Can J Anaesth 2008;55:754-60. [ Links ]
29. McKay A, Gottschalk A, Ploppa A, Durieux ME, Groves DS. Systemic lidocaine decreased the perioperative opioid analgesic requirements but failed to reduce discharge time after ambulatory surgery. Anesth Analg 2009;109:1805-8. [ Links ]
30. De Oliveira GS Jr, Fitzgerald P, Streicher LF, Marcus RJ, McCarthy RJ. Systemic Lidocaine to Improve Postoperative Quality of Recovery After Ambulatory Laparoscopic Surgery. Anesth Analg 2012;115:262-7. [ Links ]
31. Rowland M, Thomson PD, Guichard A, Melmon KL. Disposition kinetics of lidocaine in normal subjects. Ann N Y Acad Sci 1971;179:383-98. [ Links ]
32. Aps C, Bell JA, Jenkins BS, Poole-Wilson PA, Reynolds F. Logical approach to lidocaine therapy. Br Med J 1976;1:13-5. [ Links ]
33. Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain 2012;28:567-72. [ Links ]
34. Wuethrich PY, Romero J, Burkhard FC, Curatolo M. No benefit from perioperative intravenous lidocaine in laparoscopic renal surgery: a randomised, placebo-controlled study. Eur J Anaesthesiol 2012;29:537-43. [ Links ]
35. Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg 2011;62:47-58. [ Links ]
36. Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does Intraoperative Ketamine Attenuate Inflammatory Reactivity Following Surgery? A Systematic Review and Meta-Analysis. Anesth Analg 2012;115:934-43. [ Links ]
37. Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg 2004;99:482-95. Review. [ Links ]
38. Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane Review). Acta Anaesthesiol Scand 2005;49:1405-28. [ Links ]
39. Kollender Y, Bickels J, Stocki D, Maruoani N, Chazan S, Nirkin A, Meller I, Weinbrum AA. Subanaesthetic ketamine spares postoperative morphine and controls pain better than standard morphine does alone in orthopaedic-oncological patients. Eur J Cancer 2008;44:954-62. [ Links ]
40. Arroyo-Novoa CM, Figueroa-Ramos MI, Miaskowski C, Padilla G, Paul SM, Rodríguez-Ortiz P, Stotts NA, Puntillo KA. Efficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound care. Clin J Pain 2011;27:561-6. [ Links ]
41. Carstensen M, Møller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: a qualitative review of randomized trials. Br J Anaesth 2010;104:401-6. [ Links ]
42. Nesher N, Ekstein MP, Paz Y, Marouani N, Chazan S, Weinbroum AA. Morphine with adjuvant ketamine vs higher dose of morphine alone for immediate postthoracotomy analgesia. Chest 2009;136:245-52. [ Links ]
43. Savoia G, Alampi D, Amantea B, Ambrosio F, Arcioni R, Berti M, et al. Postoperative pain treatment SIAARTI Recommendations 2010. Short version. Minerva Anestesiol 2010;76:657-67. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: jusset.garcia@vhebron.net
(Jusset Teresa García-Navia).
Recibido: el 3 de junio de 2015;
Aceptado: el 9 de diciembre de 2015.