Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de la Sociedad Española del Dolor
versión impresa ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.26 no.2 Madrid mar./abr. 2019 Epub 23-Mar-2020
https://dx.doi.org/10.20986/resed.2019.3681/2018
ORIGINALS
Rapid opioid detoxification
1Médico Adjunto Servicio de Anestesiología. Cuidados Intensivos y Unidad de Dolor. Hospital General Universitario de Elda. Alicante. España.
2Coordinador de Unidad de Conductas Adictivas. UCA Departamento de Salud de Alcoi. Alicante. España.
3Jefe del Servicio de Anestesiología, Cuidados Intensivos y Unidad de Dolor. Hospital General Universitario de Elda. Alicante, España.
Introduction:
For the last 15 years we have witnessed a steady increase in opioid consumption. Being aware of an undertreatment in certain pain situations, many health care providers have encouraged their physicians to prescribe opioids to avoid unnecessary suffering. Such encouragement, also by means of switching from the traditional paper prescription to the current electronic one, has led to a wide spread in opioid prescription even among those medical specialities which never did before. Besides, new synthetic opioids with apparently less side effects, favourable kinetics and easer to take, might have arosen a wrong impression of unreal harmlessness. Therefore, the increased prescription and its obvious consequence of consumption has led to an alarming increase in the number of side effects, proving our patients not to be so well controlled. We have perceived in our Health Department several different patients with opioid consumption abuse derived from medical prescription with potential life threatening side effects, that´s why we have conducted a medical path for their detoxification.
Method and materials:
To perform our FOD path we previously admit the patients in our ICU unit. After a careful clinical, psychological, social and biological assessment, and having requested their informed consent, we monitor all their vital constants in bed and we start a deep polymodal sedation up to the required level for each patient, getting even ready for oral intubation and mechanical ventilation if needed. Our regular vital maintenance is based on fluids, deep vein thrombosis prophylaxis, digestive prophylaxis, physiotherapy, urine output and blood tests for 96 hours. Having achieved our goal, regarding the patients are stable, they are discharged to the ward for an additional 48 hours period, with psyquiatric treatment and under the care of the Addictive Conducts Unit. The patients are finally discharged from hospital with a multimodal supervision and treatment conducted by our Pain Unit, Addictive Conducts Unit and Physical Rehabilitation.
Results:
We describe the results achieved with two different drug approaches which combine different pharmacological groups frequently used for detoxification: midazolam, propofol, ketamine, clonidine and naloxone, for our aim of succeeding in keeping the patients opioid-free without endangering their haemodynamic, breathe or biology.
Conclusions:
FOD has proved to be a successful treatment in rescuing the patients from a living hell out of which they would have found it impossible to leave without qualified help. We deem it safe with the right ICU surveillance, since no major complications have occurred, but a thereafter following and help is mandatory, since, like any other patient attended at a Pain Clinic, they require a favouring social and familiar environment to avoid any relapse. Finally, and given our results, we consider this detoxification method right and safe but highly costly in resources.
Key words: Opioids; opioids abuse; fast opioid detoxification; FOD
INTRODUCTION
The use of long-term opioid therapy for non-cancer chronic pain (NCCP) has increased significantly in recent decades, as well as the subsequent introduction of rapid-release opioid formulations in these cases could have produced an increase in cases of opioid addiction in patients with NCCP 1. The proportion of patients with iatrogenic addiction to opioids is very difficult to estimate due to the absence of definitions and specific criteria for patients with chronic pain and opioid use 2.
The criteria used for the diagnosis of opioid consumption disorder (OCT) are based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition (DSM-5) 3.
The DSM-5 defines the opioid use disorder (OUD) as a set of cognitive, behavioral and psychological symptoms that lead an individual to the continued use of a substance despite the problems related to its use 4.
The abuse of opioids produces alterations in brain circuits, which are the underlying causes for the development of dependency and addiction to these substances. Dependence refers to the imperative need to continue the use of opioids to avoid withdrawal syndrome, and addiction is defined as a chronic and recurrent disease of the brain characterized by the pursuit and compulsive use of substances despite their harmful consequences, inability to stop using the substance, neglecting work, social or family obligations and sometimes, depending on the substance, tolerance and deprivation or withdrawal.
Risk factors associated with an increase in the inappropriate use of opioid analgesics when prescribed for the management of chronic pain, such as substance abuse disorder, family history of substance abuse, associated mental illness, history of legal problems or jail sentences, white race and under 40-45 years of age, have been described 5.
In the pain units, patients referred by other specialties are treated. These patients are diagnosed of various pathologies, with chronic pain in general valued from moderate to severe, which in its evolution require the use of opioids. These treatments can potentially cause addition symptoms. Therefore, an exhaustive follow-up to detect the cases that could develop such symptoms is required. This follow-up should be conducted by the professionals specialized in the treatment of pain.
Other important concepts to consider are tolerance and dependence. Tolerance is the state of adaptation in which an increase in the doses of the opioid is required to obtain the desired effect, or there is a decrease in the effect of the substance over time 6.
The continued use of opioids for pain management creates dependence. An alteration in the physiological response resulting from the adaptation of the opioid-receptor binding due to chronic use occur in the physical dependence on opioids. This chronic use is characterized by the presence of OWS occurring after abrupt cessation, rapid reduction, decrease in level of the drug in blood and/or the administration of an antagonist 7. It is important to differentiate this term of addiction, which describes a chronic neurobiological disorder that involves an aberrant use of the opioid and a maladaptive social behavior that implies a loss of self-control that leads to compulsive and often self-destructive use 8.
In the present article we describe six cases of rapid opioid detoxification (ROD) conducted in the recovery unit of our hospital, between 2011 and 2016, of patients showing symptoms of opioids addiction.
ROD: DETOXIFICATION METHOD
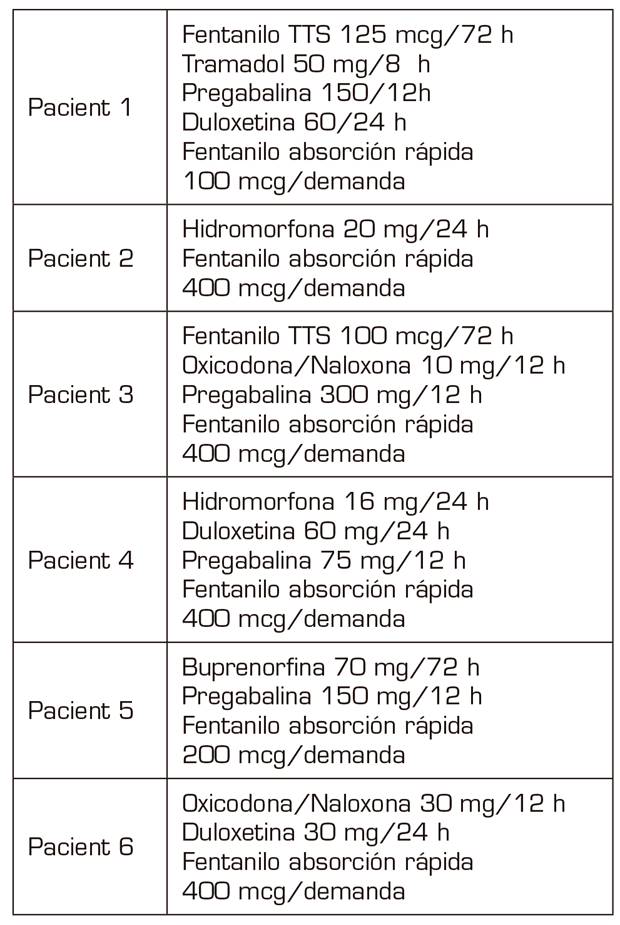
All the patients had been diagnosed with neuropathic pain, of years of evolution, with values > 7 regarding pain intensity in the visual analogue scale (VAS), in which different neuromodulators and opioids had been used from lower to higher, as indicated the World Health Organization (WHO) analgesic ladder (Table I).
They are two women and four men aged 36-53 years (in the year in which the ROD was performed) socially integrated with relatives who supported them, and without medical history that contraindicated the detoxification.
All the cases described in the present study were diagnosed in the consultation of the Pain Unit at our Health Department (220,000 inhabitants). Four of them referred from primary care and after being treated in our unit and two of them after referral from primary care with treatments already established by their general practitioners.
All of them had a point in common, which consisted in the use of fast-acting fentanyl for the treatment of non-cancer breakthrough pain some time before they began to increase their care demands for its prescription, since its consumption was increasing not only regarding the daily doses administered but also regarding the amount (micrograms) prescribed. This fact, together with the appearance of agitation and aggressiveness in the consultations and the denial that their baseline treatment was effective, verified by the anesthesiologists, by our nurse who answers telephone consultations in the PTU, as well as by the relatives, were the symptoms that led us to diagnose the addiction syndrome and to suggest a solution 4.
After the diagnosis of addiction syndrome (DSM-5 level 3, severe OUD), an appointment was booked for each patient that should be accompanied by a close relative. They were thoroughly informed about the addiction the patient suffered and the possibility of entering the recovery unit, where patients would be treated for a few days, the drugs that they would be using, the monitoring of vital signs and the risks. All 6 patients accepted and signed an informed consent together with their family member and an anesthesiologist from our unit.
METHOD
All the patients were admitted in a fasted state in the morning of a Monday. A peripheral vein was catheterized, a nasogastric (NG) tube and a urinary catheter were placed and blood was collected for a baseline analysis consisting of a blood count, biochemistry and coagulation determinations.
Non-invasive blood pressure, ECG, O2 saturation, diuresis and hourly temperature were monitored.
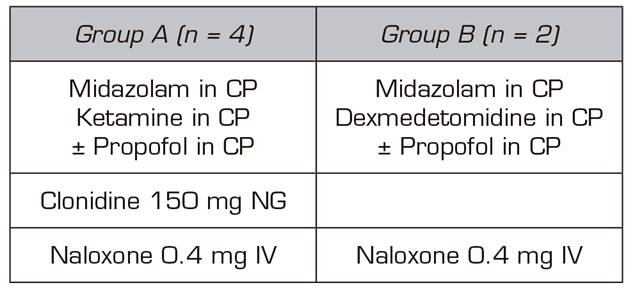
Two treatment groups were established (Table II).
In four of the patients, the basic treatment consisted of an infusion of ketamine and midazolam plus propofol, which was reserved as a drug to be used, if after hours of admission without the administration of any opioid the patient needed a deeper sedation. These four patients were administered 150 mg 1 tablet NG clonidine every 12h, SC enoxaparin every 24h at prophylactic doses for deep vein thrombosis (DVT) and 40 mg IV omeprazole every 24 hours. For the symptoms and signs expected in opioid withdrawal (Table III) (such as hypertension, diarrhea, muscle-ages, vomiting, anxiety, etc.), we protocolized the administration of labetalol, loperamide, paracetamol/desketoprofen and ondansetron, depending on the appearance of those symptoms and signs, as long as there were no individual contraindications for their use.
Dexmedetomidine and midazolam were used for sedation during the detoxification process of the other two patients. Similarly, to the first four patients, propofol was used to increase the level of sedation only when the signs of withdrawal began. Furthermore, patients also underwent gastric protection, DVT prophylaxis with low molecular weight heparin due to prolonged immobilization, but in the latter two cases clonidine was not used orally, because clonidine is a molecule acting at the central nervous system on the same receptors as dexmedetomidine 9.
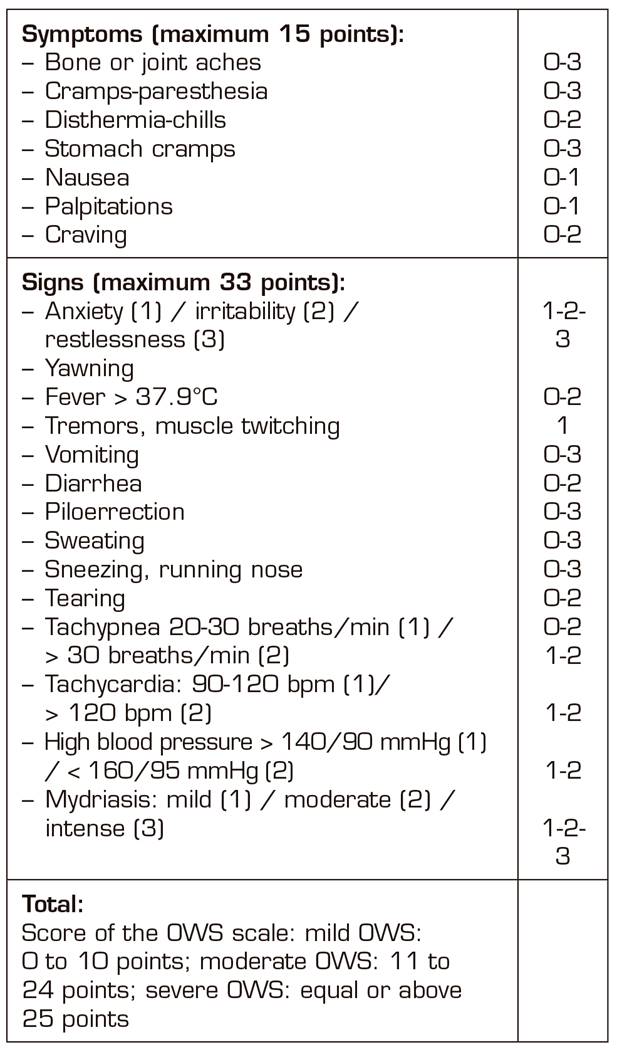
All patients, during the process, were assessed using the rating scale of the opioids withdrawal syndrome (OWS) until signs of detoxification ceased (Table III) 10.
RESULTS
Doses used were always individualized according to the needs of each patient and in increasing doses, according to their needs:
- Midazolam: perfusion started from 0.05 to 0.1 mg/kg/h, reaching maximum doses of 7 mg/h.
- Ketamine: started at 0.1 mg/kg/h and reached maximum doses of 20 mg/h.
- Dexmedetomidine: started at 0.2 μg/kg/h and reached doses of 1.4 μg /kg/h.
- Propofol: started as needed by the patients and their doses ranged from 1 mg/kg/h to 2 mg/kg/h.
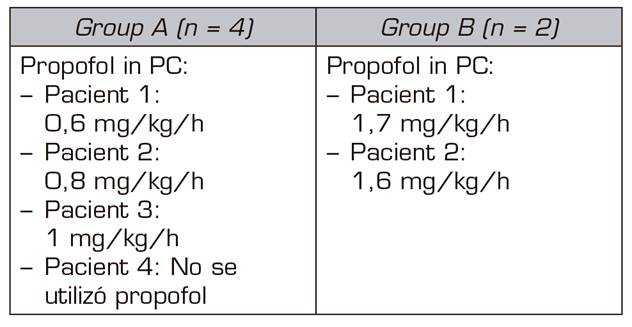
The two patients treated with midazolam and dexmedetomidine required perfusion of propofol between 10 and 12 hours after the beginning of the detoxification process; while only one case out of the 4 patients treated with midazolam, ketamine and clonidine did not require perfusion of propofol. Perfusion of propofol began later, between 30 and 36 h after the beginning of the process of detoxification, in the other 3 patients.
In the 4 cases in which the sedation consisted of an infusion of midazolam and ketamine, the signs of the OWS were less marked than in the two cases in which we used infusions of midazolam and dexmedetomidine. In addition, the perfusion doses of propofol in the first 4 cases were lower than in those patients of the midazolam-dexmedetomidine group (Table IV).
Both ketamine and dexmedetomidine infusions were not maintained for more than 72 hours, and they were always progressively reduced until discontinuation, maintaining the perfusion of midazolam and propofol, which subsequently decreased gradually until discontinuation.
Regarding the cessation of symptoms and signs of the OWS, these occurred between 72 and 96 hours in all the cases studied. Then a dose of 0.4 mg IV naloxone was administered and the responses of each patient were observed, without any of the cases showing signs of opioid deprivation.
The length of the stay at the recovery unit was five days for all patients, except for one case who stayed at the unit for 8 days. In the latter case, the patient was also addicted to nicotine and with a history of moderate associated alcoholism. There was no case of respiratory depression or severe pain or psychomotor agitation that required intubation of the patient.
The patients are usually discharged on the fifth day to the conventional hospitalization ward, where they stayed for 48 hours undergoing alternative treatment, and monitored in case any type of complication occurs.
After discharge, treatments with neuromodulators, olanzapine and in two cases also with THC (tetrahydrocannabinol) in sublingual spray for crisis of neuropathic pain began, in case.
All the patients, after the detoxification, were referred to the unit of addiction behavior for following-up the progress and continued with the periodic checkup in our unit.
DISCUSSION
Opioid addiction is a complex disease that is difficult to treat. The treatment is divided into three processes: stabilization, detoxification and maintenance therapy 11. Stabilization consists in the substitution of the opioid for treatments that ensures that the use of the drug is independent of the mental state and the circumstances. Detoxification consists in the withdrawal of the opioid safely and effectively, minimizing the withdrawal syndrome (with opioid receptor agonist drugs, such as methadone or buprenorphine or with agonists of alpha-2 adrenergic receptors such as clonidine or dexmedetomidine). Maintenance therapy consists of relapse prevention (usually with the administration of opioid receptor antagonists, such as naloxone or naltrexone).
The problems that clinical experience has helped us to observe is that, since the introduction of treatments outside of the data sheet (non-cancer patients) of the fast-acting fentanyls, the "fascination" that produces the effect of the drug through areas of brain stimulation (with great feeling of gratification) is greater than analgesia in at the beginning. This means that almost all of our non-cancer patients, included in the FOD program, tend to abandon the baseline treatment, opioids, adjuvants, etc., in favor of performing analgesia mediated by the rapid opioid exclusively and in doses absolutely larger than those recommended by their doctor, both in amount per dose and in frequency of them. In fact, this is the main important recommendation we make: a comprehensive pharmacotherapeutic follow-up of patients who are treated with fast-acting fentanyl out of the datasheet, as well as the performance of a pact of time and evaluation of therapeutic efficacy while evaluating other alternatives that would allow an early withdraw of these drugs. In general, we can say that the incidence of overdose and iatrogenic problems with this fast-acting fentanyl is almost non-existent when prescribed and controlled from our Pain Unit, but not when prescribed by other medical specialties, with less knowledge of the problem generated by the chronic use of opioids.
The traditional management in the 1970s of this syndrome implied either replacement by a long-acting opioid such as methadone and its subsequent gradual withdrawal, or the non-use of opioids (clonidine with adjuvants such as analgesics, hypnotics and benzodiazepines). Generally, in both processes, a mu-receptor antagonist such as naltrexone was gradually introduced 12,13. The rate of failure and relapse in both cases was high due to the discomfort and distress that the patients experienced during the procedure. Therefore, in the 1980s, Loimer et al. 14developed for the first time this process of detoxification with general anesthesia and intubation, based on Yale University publications 15on ROD methods.
From then, different researchers have been introducing improvements and modifications to the ultra-ROD technique 16.
Studies on the efficacy of gradual opioid withdrawal methods using methadone or buprenorphine treatment versus new methods of ROD and ultra-ROD have not been able to demonstrate the clear benefits of one technique or another. Clinicians should be guided by the response of patients to determine the duration of the opioid withdrawal period 17.
Johnson and Carr 18, proposed the following classification in 2003:
- In the ultra-ROD, the use of general anesthesia can be used for less than 6 hours.
- In the ROD, a deep sedation varying from 6 to 72 hours in the studies described.
Several studies have described rapid detoxification techniques over the years, all using potent anesthetic agents that induce deep hypnosis to mitigate the symptoms of the OWS. It is not known exactly by which mechanisms anesthetic agents are able to block the expression of opioid withdrawal, but one of the explanations could be in the interference of these drugs with glutamate, which is closely associated with a noradrenergic hyperactivity that in part is behind the pathophysiology of the withdrawal syndrome 19.
The efficacy results for ultra-ROD are not clear and, therefore, recommendations on their use are controversial. In a recent review published in 2015, ultra-ROD techniques are not recommended under general anesthesia due to the registry of severe complications in the literature, including cardiac arrest and death 20,21. In a systematic review of 5 randomized studies, they conclude that its use is not advisable due to the lack of benefit and the potential documented risks and the high costs that generate the admission of patients in intensive care units based on general anesthesia or deep sedation 22.
Another point to discuss is the different drugs that we have used in the development of our ROD protocol.
In opioid detoxification techniques through induction of general anesthesia, the use of drugs such as clonidine and dexmedetomidine is approved, drugs that are used in a protocolized manner in our intensive care unit. We describe below the pharmacological reasons for which these drugs are effective, although their indication is out of the data sheet.
Due to the high noradrenergic activity that is triggered after the withdrawal of opioids (characteristic withdrawal syndrome), the use of α2 agonists, such as clonidine or dexmedetomidine, has been successful in a large number of published studies. Their main limitation to use is the antihypertensive effect. Their beneficial effects in this task are their sedative activity, the reduction of the activation of the sympathetic nervous system and the decrease in the requirements of opioids described by mechanisms poorly known but that seem to be related to their activity on the nucleus ceruleus.
The α2 agonist drugs are usually combined with another type of drugs, which we also use in a protocolized way in our study. These drugs are benzodiazepines, in our case continuous perfusion (CP) of midazolam, ideal for reducing levels of anxiety and improvement of sedation levels, ondansetron for the reduction of nausea and vomiting and loperamide for the treatment of diarrhea in patients with withdrawal syndrome. Other drugs used in our study are propofol, an hypnotic drug that in CP controlled by Target Control Infusion (TCI) is ideal for sedation to maintain spontaneous ventilation, and ketamine, whose sympathetic activity could be conflicting in its use for opioids detoxification but, as we observed in our study, ketamine is useful in combination with the rest of the drugs used due to its analgesic potency.
Ketamine is a drug widely used in anesthesia. Ketamine is classified as an NMDA receptor antagonist but has many other actions on mu, delta and kappa opioid receptors, and also Ketamine inhibits the reuptake of serotonin, dopamine and norepinephrine 23. This mechanism of action has been used in anesthesia to decrease tolerance to opioids, reduce the consumption of analgesics and increase the time in which patients begin to consume analgesics in the postoperative period 24. Moreover, the use of ketamine makes sense in patients who have established chronic pain.
Previous studies have described a successful opioid detoxification with ketamine, even with ketamine orally 23. In a group of 58 patients, the administration of continuous ketamine infusions in subanesthetic doses, 0.5 mg/kg/h dose, was also successful. The group treated with ketamine experienced better control of OWS 25. These data are consistent with the findings observed in our study that, despite the limitations of the sample size, the group treated with ketamine presented fewer signs and symptoms associated with the withdrawal syndrome due to opioid deprivation and lower use of propofol.
Our medium- and long-term results on the treated patients reaffirm us in the need to make the patient see:
The continued risk for relapse that patient will have throughout life.
That patient must inform the anesthesiologist immediately if he/she is going to have surgery.
That patient will be monitored frequently by specialists in pain, addictive behaviors, psychiatry and psychology, given that patient has overcome a critical illness and the health of the patient should be monitored.
In general, our patients report that they have got a "second chance" at life. They continue with symptoms of pain but, in general, they refer less intense pain than prior the treatment, but above all they report having gained infinitely in quality of life, since they were immersed in the spiral of side effects of opioids at all levels, worsening even the painful perception severely. We believe that these patients will need analgesic/adjuvant treatment, perhaps for life, but our limitation in the number of patients and the time after treatment does not allow us to know the long-term development.
Unfortunately, we have had a therapeutic failure in a young patient. After an extraordinary initial success in this patient with an improvement in quality of life and absence of pain (which also encouraged her to want to create a support group for patients with similar conditions to prompt them to undergo ROD). After 6 months, this patient began to reject everything, being evaluated by our entire multidisciplinary care group without being able to overcome the relapse, she began to take opioids out of the prescription of our environment, moving away from the guided and recommended therapy for her.
CONCLUSIONS
- According to the duration of our opioid detoxification method, we could classify it within the group defined as ROD.
- In all cases, a good level of sedation has been maintained (measured by the Richmond Agitation Sedation Scale (RASS)) without requiring invasive assistance of the airway and maintaining spontaneous ventilation.
- No patient in the study had severe complications. Only a minor bronchoaspiration event occurred in one patient without clinical consequences.
- The amnesia of the patients of almost the entire length of the stay at our intensive care unit is noteworthy, even the period they were awake and collaborating with the nursing staff.
- The two cases that required the highest use of drugs were those that had the highest opioid use at baseline. These were treated in the group of dexmedetomidine and midazolam, without ketamine, a fact that should be noted for the analgesic and hypnotic effect of ketamine. In addition, patients in this line of treatment showed more symptoms and signs of OWS. These data are merely observational due to the small sample size of our study.
- Studies with larger sample size are needed to achieve conclusions with more quality and scientific evidence.
- We strongly recommend selecting patients very well to perform this technique. Thereby, they would be able to assimilate the effort and risk they are going to undergo, so that they become aware that a drastic change in their life is going to take place, and accept it.
- We sincerely believe that the iatrogenesis due to the poorly controlled use of opioids should be solved with more training on its use, and the early detection of patients susceptible to developing problems with these drugs.
- We think that this technique represents "a second chance" from which selected patients can benefit.
REFERENCES
1. Manchikanti L, Fellows B, Ailinani H. Therapeutic use, abuse and nonmedical use of opioids: a ten-year perspective. Pain Physician 2010;13(5):401-35. [ Links ]
2. Manchikanti L, Benyamin R, Datta S, Vallejo R, Smith H. Opioids in cronic non-cancer pain. Expert review of neurotherapeutics 2010;10(5):775-89. DOI: 10.1586/ern.10.37. [ Links ]
3. Ballantyne JC, Sullivan MD, Kolodny A. Opioid Dependence vs. Addiction: A Distinction Whithout a Difference?. Archives of Internal Med 2012;172(17):1342-43. DOI: 10.1001/archinternmed.2012.3212. [ Links ]
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V). American Psychiatric Pub 2013. DOI: 10.1176/appi.books.9780890425596. [ Links ]
5. Becker W, Starrels J. Prescription drug misuse: Epidemiology, prevention, identification and management. Update 2015. [ Links ]
6. Robinson RC, Gatchel RJ, Polatin P, Deschner M, Noe C, Gajraj N. Screening for problematic prescription opioid use. The Clinical journal of Pain 2001;17(13):220-8. [ Links ]
7. Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: evaluation towards universal agreement. Journal of Pain and symptom management 2003;26(1):655-67. [ Links ]
8. Brill S, Ginosar Y, Davidson EM. Perioperative management of the chronic pain patient with opioid dependency. Curr Opin Anaesthesiol 2006;19(3):325-31. DOI: 10.1097/01.aco.0000192813.38236.99. [ Links ]
9. Alfonso J, Reis F. Dexmedetomidina: rol actual en anestesia y cuidados intensivos. Rev Bras Anestesiol 2012;62(1):118-33. [ Links ]
10. Wang RIH, Wiesen RL, Lamid S, Byung LR. Rating the presence and severity of opiate dependence. Clin Pharmacol Ther 1974;16(4):653-8. [ Links ]
11. Praveen KT, Law F, O'Shea J, Melichar J. Opioid dependence. BMJ Clin Evid 2011. pii: 1015. [ Links ]
12. Blachley P, Casey D, Marcel L, Demey DD. Rapid detoxification from heroin and methadone using naltrexone. A model for the treatment of the opiate abstinence syndrome. Develompents in the field of drug abuse. Cambridge MA: Schenkman Publishing Co; 1975. [ Links ]
13. Kurland AA, McCabe L. Rapid detoxification of the narcotic addict with naloxone hydrochoride. A preliminary report. J Clin Pharmacol 1976;16(1):66-74. [ Links ]
14. Loimer N, Schmidt R, Presslich O, Lenz K. Continuous naloxone administration suppresses withdrawal symptoms in human opiate addicts during detoxification treatment. J Psychiatry Res 1989;23(1):81-6. [ Links ]
15. Riordan CE, Kleber HD. Rapid opiate detoxification with clonidine and naloxone. Lancet 1980;1(8177):1079-80. [ Links ]
16. Singh J, Basu D. Ultra-rapid opioid detoxification: current status and controversies. J Postgrad Med 2004;50(3):227-32. [ Links ]
17. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med 2015;9(5):358-67. DOI: 10.1097/ADM.0000000000000166. [ Links ]
18. Johnson TS, Carr M. Naltrexona mediated opiate detoxification: a matter of terminology. Addict Biol 2003;8(3):267-9. DOI: 10.1080/13556210310001613471. [ Links ]
19. Streel E, Verbanck P. Ultrarapid Opioid Detoxification: from clinical application to basic science. Addict Biol 2003;8(2):141-6. DOI: 10.1080/1355621031000117365. [ Links ]
20. Hamilton RJ, Olmedo RE, Shah S, Hung OL, Howland MA, Perrone J, et al. Complications of ultrarapid opioid detoxification with subcutaneous naltrexone pellets. Acad Emerg Med 2002;9(1):63-8. [ Links ]
21. Centers for Disease Control. Deaths and Severe Adverse Events Associated with Anesthesia-Assisted Rapid Opioid Detoxification: New York City, 2012. Morbidity and Mortality Weekly. [ Links ]
22. Gowing L, Ali R, White JM. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database Syst Rev 2010. [ Links ]
23. Lalanne L, Nicot C, Lang JP, Bertschy G, Salvat E. Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: a case report. BMC Psychiatry 2016;16(1):395. DOI: 10.1186/s12888-016-1112-2. [ Links ]
24. Radvansky BM, Shan K, Parikh A, Sifonios AN, Le V, Eloy JD. Role of ketamine in acute postoperative pain management: a narrative review. Biomed Res Int 2015;2015:749837. DOI: 10.1155/2015/749837. [ Links ]
25. Jovaisa T, Laurinenas G, Vosylius S, Sipylaite J, Badaras R, Ivaskevicius J. Effects of ketamine on precipitated opiate withdrawal. Medicina (Kaunas) 2006;42(8):625-34. [ Links ]
Received: April 26, 2018; Accepted: November 14, 2018











 texto en
texto en