Introduction
Super-response (SR) to Cardiac Resynchronization Therapy (CRT) was first defined by Blanc et al1 in a subgroup of patients without ischemic cardiomyopathy who normalized left ventricular function after CRT implantation. On long-term follow-up, these patients present a better prognosis and less appropriate intracardiac-shocks than other CRT-patients2,3. Even though these patients are of great interest, there still is a lack of agreement regarding its definition, which can be defined as a clinical or an echocardiographic improvement, or a combination of the two4. Accordingly, predictors of SR present a great variability. Non-ischemic cardiomyopathy, female sex, body mass index < 30kg/m2, small cardiac dimensions, long QRS duration, and left bundle branch block (LBBB)5, as well as greatest QRS reduction at follow-up6, absence of ventricular tachycardia and previous right ventricular pacing7 have been identified as predictors of SR.
Furthermore, there is an absence of information about the contribution of cardiac biomarkers in predicting SR. Cardiac biomarkers such as Interleukin 6 (IL-6), Tumor Necrosis Factor α (TNFα) and soluble CD40 Ligand (sCD40L), as well as N-Terminal pro B-type Natriuretic Peptide (NT-proBNP), high sensitive Troponin T (hsTnT), high sensitive C-reactive Protein (hsCRP) and Galectin-3 (Gal-3) might play a role in predicting SR. They are released in myocardial injury (hsTnT), are activated due to the neuro-hormonal cascade (NT-proBNP), or participate in cardiac remodeling: activating inflammation (hsCRP, IL-6, TNF-α, SCD40L) or promoting myocardial, vascular and valvular (aortic) fibrosis (Gal-3)8. The identification of one or more of these biomarkers in the prediction of SR could be of great value.
The aim of this study is primary to assess the role of these cardiac biomarkers in predicting SR. Secondarily, to identify other clinical, electrocardiographic and echocardiographic predictors of SR.
Methods
Patients attended at the Department of Cardiology of the Clínica Universidad de Navarra between January 2010 and March 2012 with indication of CRT implantation and NYHA II-IV were prospectively enrolled. Patients with permanent RV-Pacing or atrial fibrillation and LVEF ≤35% were also included. Exclusion criteria were: age < 18 years-old, decompensated heart failure, percutaneous coronary intervention or coronary artery bypass grafting within the first 3 months and myocardial infarction within 40 days. Patients with genetic and congenital heart diseases were also excluded. The investigation conformed with the principles outlined in the Declaration of Helsinki of 1975 and the revision of October 2000. The ethic committee of the institution approved the study protocol and informed consent was obtained from all patients prior to participation.
SR was defined as a reduction in left ventricular end- systolic volume (LVESV) ≥ 30%9,10 at one year follow-up. All other patients, including those who died or underwent heart transplant, were considered non-SR. Clinical and electrocardiographic characteristics were obtained from the medical records. Minnesota Living with Heart Failure Questionnaire (MLWHFQ) and six- minute walking test (6MWT) were also performed at baseline and after 12 months.
All CRT devices were implanted using an intravenous access. The number of implanted pacing leads depended on cardiac rhythm (three if sinus rhythm, two if atrial fibrillation). In patients who underwent an upgrade of the stimulation system, a CRT-device and a pacing lead on the left lateral wall were implanted. CRT with implantable cardiac defibrillator (CRT-D) was implanted in patients with ischemic heart disease or dilated cardiomyopathy, which were expected to survive substantially longer than one year with good functional status.
Two-dimensional and real-time 3-dimentional (RT3D) echocardiographies were performed at baseline and twelve months after the implant. All tests were performed using an IE33 echocardiograph (Philips Medical System, Bothell VVA, USA). A 3.5 MHz and a 3D transducer (X3-1) were used. Images were obtained according to standard views and stored in an external drive. Measurements were analyzed off-line by two independent experienced echocardiographists. After measuring function and left ventricular (LV) dimensions, we proceeded to measure mechanical dyssynchrony. Mechanical dyssynchrony was assed at:
- intraventricular level: septal-posterior wall motion delay, SPWMD, measured using M-mode in the parasternal short axis view, and systolic dyssynchrony index, SDI, measured using RT3D echocardiography by apical window and ECG-gated acquisition, with at least 4 QRS-intervals and one breath hold.
- atrio-ventricular level: AV-dyssynchrony, measured as the time of blood-flow across the mitral valve divided by the time between two consecutive QRS-complexes.
- interventricular level: (interventricular mechanical delay, IVD), measured as the difference between left and right ventricular preejection intervals measured by pulsed Doppler.
Serum and heparin blood samples were obtained between twelve and one hour before CRT-implantation, after at least 30 minutes fasting and in supine position. After extraction, aliquots were stored at -20°C. Analyses were made simultaneously after recruiting all samples in order to avoid intra-assay variability. The origin of the samples was unknown for the examiner. IL-6, hsTnT, hsCRP and NT-proBNP were analyzed by electrochemiluminescence immunoassay (ECLIA) autoanalyzer Cobas 8000 (Roche Diagnostics). TNFα and SCD40L were quantified by enzyme immunoassay following the manufacturer's instructions (R & D systems). Gal-3 was measured by fluorescence enzyme immunoassay (FEI) with a Minividas analyzer (Biomerieux).
For the statistical analysis we considered a significant p value of < 0.05. Statistical analyses were performed using SPSS 15.0 (SPSS Inc, Chicago, Illinois). Descriptive analysis of qualitative parameters were expressed as frequency and percentage. Quantitative variables were expressed as mean value ± standard deviation (SD). Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess normality of the variables. Parametric and non-parametric tests (Chi-square, Fisher exact test, Student's-t, Mann-Whitney U) were used to determine relation or interaction between the different variables. Uni- and multivariate logistic regression was used to analyze qualitative variables by odds ratio (OR) and its 95% confident interval (95%CI). Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of the parameters by means of their area under curve (AUC) and 95%CI.
Results
At the end of the follow-up period, 50 patients were included and 23 patients (46%) were identified as SR according to the reduction in LVESV. A CRT-D was implanted in 27 patients (54%) (11 SR vs. 16 non-SR, p = 0.4).
Table 1 shows baseline characteristics of the study population. At the time of enrolment, we found that female, absence of ischemic cardiomyopathy, absence of lateral, inferior and posterior infarctions, longer QRS-width and LV-lead in lateral medial position were more often associated to SR.
Table 1. Clinical, demographic and electrocardiographic characteristics at baseline and electrocardiographic characteristics directly after CRT implantation
| Characteristics | SR (n = 23) | Non- SR (n = 27) | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (years)* | 72.2 ± 11.2 | 74.5 ± 9.6 | 0.45 |
| Female sex | 8 (34.8) | 3 (11.1) | 0.04 |
| Follow-up (months) | 13.5 ± 9.2 | 8.7 ± 6.2 | 0.05 |
| 6-minute walking test (m)* | 278.3 ± 129.1 | 265.4 ± 115.5 | 0.66 |
| MLWHFQ (score)* | 37.7 ± 22.6 | 41.7 ± 20.3 | 0.57 |
| NYHA functional class | |||
| II | 10 (43.5) | 7 (25.9) | 0.19 |
| III / IV | 13 (56.5) | 20 (74.1) | |
| HF hospitalizations* | 1.4 ± 2.2 | 1.7 ± 1.6 | 0.17 |
| Ischemic cardiomyopathy | 3 (13.0) | 17 (62.9) | < 0.001 |
| Smoker | 11 (47.8) | 14 (51.8) | 0.25 |
| Arterial hypertension | 15 (65.2) | 20 (74.1) | 0.49 |
| Diabetes mellitus | 6 (26.1) | 12 (44.4) | 0.18 |
| Hypercholesterolemia | 10 (43.5) | 14 (51.8) | 0.55 |
| Familiar history of IC | 1 (4.3) | 2 (7.4) | 0.65 |
| Hyperuricaemia | 7 (30.4) | 6 (22.2) | 0.50 |
| Location infarction | |||
| Anterior | 3 (13.0) | 8 (29.6) | 0.15 |
| Lateral | 0 | 5 (18.5) | 0.03 |
| Inferior | 1 (4.3) | 9 (33.3) | 0.01 |
| Posterior | 0 | 6 (22.2) | 0.01 |
| Septal | 2 (8.7) | 6 (22.2) | 0.19 |
| Apical | 2 (8.7) | 4 (14.8) | 0.5 |
| Previous pacemaker | 9 (39.1) | 5 (18.5) | 0.1 |
| Atrial fibrillation | 9 (39.1) | 17 (62.9) | 0.09 |
| Previous VT | 0 | 4 (14.8) | 0.054 |
| LBBB | 12 (52.2) | 16 (59.2) | 0.38 |
| QRS duration (ms)* | 157.7 ± 22.9 | 140.8 ± 19.2 | 0.01 |
| PR duration (ms)* | 177.0 ± 39.9 | 213.9 ± 50.9 | 0.15 |
| Treatment | |||
| ACEI | 12 (52.2) | 11 (40.7) | 0.42 |
| ARB | 6 (26.1) | 10 (37) | 0.41 |
| Beta-blockers | 13 (56.5) | 20 (74.1) | 0.19 |
| Aldosterone antagonists | 8 (34.8) | 9 (33.3) | 0.9 |
| Diuretics | 17 (73.9) | 23 (85.2) | 0.32 |
| Digoxin | 8 (34.8) | 13 (48.1) | 0.34 |
| Antiarrhythmics | 6 (26.1) | 2 (7.4) | 0.07 |
| Statins | 9 (39.1) | 11 (40.7) | 0.9 |
| ASA | 4 (17.4) | 7 (25.9) | 0.07 |
| Clopidogrel | 2 (8.7) | 0 | 0.5 |
| ASA+ Clopidogrel | 4 (17.4) | 4 (14.8) | 0.08 |
| Oral anticoagulation | 13 (56.5) | 15 (55.5) | 0.9 |
| Post-implantation | |||
| AV-node ablation | 7 (30.4) | 6 (22.2) | 0.5 |
| lateral medial position | 16 (69.6) | 7 (25.9) | 0.002 |
| implant crt-d | 11 (47.8) | 16 (59.2) | 0.42 |
SR: super-response; *: mean ± standard deviation; MLWHFQ: Minnesota Living With Heart Failure Questionnaire; NYHA: New York Heart Association; HF: heart failure; IC: ischemic cardiomyopathy; VT: ventricular tachycardia; LBBB: left bundle branch block; PR: distance between the P wave and the Q wave on the electrocardiogram; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; ASA: acetylsalicylic acid; AV: atrio-ventricular; CRT-D: cardiac resynchronization therapy with defibrillator.
Up to nine patients (17.6%) died during follow-up, six cardiac and three non-cardiac deaths; four of these patients had a CRT-D implanted (p = 0.5). Patients who died were significantly older (77.1 ± 4.0 vs. 72.7 ± 10.9 years, p = 0.04), had more ischemic cardiomyopathy (7 vs. 14 patients, p = 0.01), especially lateral (p = 0.009) and posterior infarctions (p = 0.003), had worse NYHA class (8 out of 9 patients in NYHA III / IV, p = 0.018) and were more frequently hospitalized because of HF previous to CRT implant (2.0 ± 1.58 vs. 1.4 ± 1.9, p = 0.007) compared to survivors. Ischemic cardiomyopathy significantly increased the risk of death (OR = 1.8, 95%CI: 1.2-29.4, p = 0.024).
At one year follow-up, no clinical significant differences were seen between SR and non-SR. Up to 21 SR and 16 non-SR had a NYHA functional class of I or II, and two SR and two non-SR had a NYHA functional class III or IV (p = 0.24). Mean 6MWT was 377.23 ± 132.71 m in SR vs. 397.57 ± 114.26 m in non-SR (p = 0.81), and the mean MLWHFQ score was 20.45 ± 19.9 points in SR vs. 23.18 ± 20.34 points (p = 0.49). Hospitalizations during the observation period were also similar between groups, as one SR was hospitalized four times and four non-SR were hospitalized one time each (0.17 ± 0.83 in SR vs. 0.21 ± 0.42 in non-SR, p = 0.12). Survival free from hospitalizations was 95.7% in SR and 85.2% in non-SR. Electrocardiographically, at follow-up, SR had narrower QRS width (126.34 ± 21.23 vs. 132.89 ± 17.1 ms), but the differences were not statistically significant (p = 0.12). There was only one ICD-shock during follow-up in a SR who received a CRT-D as a primary prophylaxis. This patient had a very reduced LVEF at baseline (13%) and improved up to 27% at follow-up.
Preimplant echocardiographic parameters are shown in Table 2. Absence of mitral regurgitation was more often seen in SR (11 vs. 6 patients, p = 0.04). Regarding mechanical dyssynchrony, SR showed more prolonged IVD compared to non-SR, but the difference was not statistically significant (p = 0.06).
Table 2. Echocardiographic parameters (mean ± standard deviation) preimplant and at 12 months follow-up
| Preimplant | p | Follow-up | p | |||
|---|---|---|---|---|---|---|
| SR (n = 23) | Non-SR (n = 27) | SR (n = 23) | Non-SR (n = 18) | |||
| LVEF (%) | 28.4 ± 7.4 | 27.4 ± 7.1 | 0.6 | 49.2 ± 11.9 | 40.6 ± 9.3 | 0.02 |
| LVEDV (ml) | 187.4 ± 51.7 | 200.7 ± 66.0 | 0.5 | 130.3 ± 34.4 | 202.4 ± 65.8 | < 0.0001 |
| LVESV (ml) | 132.2 ± 41.7 | 144.4 ± 60.7 | 0.6 | 70.44 ± 27.5 | 123.4 ± 50.26 | < 0.0001 |
| LVEDD (mm) | 65.9 ± 9.8 | 65.4 ± 10.9 | 0.8 | 55.9 ± 8.9 | 63.8 ± 6.5 | 0.01 |
| LVESD (mm) | 54.8 ± 10.3 | 54.6 ± 12.1 | 0.9 | 40.5 ± 8.9 | 47.9 ± 7.2 | 0.008 |
| Mitral regurgitation, n (%) | ||||||
| absent | 11 (47.8) | 6 (22.2) | 0.04 | 9 (39.1) | 3 (16.7) | 0.02 |
| mild | 8 (34.8) | 14 (51.8) | 0.2 | 13 (56.5) | 13 (72.2) | 0.55 |
| moderate | 4 (17.4) | 5 (18.5) | 0.6 | 0 | 2 (11.1) | 0.1 |
| severe | 0 | 2 (7.4) | 0.2 | 0 | 0 | |
| Tei Index | 1.1 ± 0.5 | 0.9 ± 0.6 | 0.2 | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.2 |
| IVD (ms) | 41.1 ± 21.4 | 26.8 ± 19.7 | 0.06 | 17.8 ± 19.1 | 16.1 ± 19.7 | 0.7 |
| AV dissynchrony | 0.45 ± 0.3 | 0.4 ± 0.1 | 0.8 | 0.44 ± 0.1 | 0.5 ± 0.07 | 0.5 |
| Septal-posterior delay (ms) | 147.9 ± 89.5 | 155.6 ± 55.9 | 0.5 | 42.8 ± 42.9 | 45.6 ± 31.9 | 0.5 |
| SDI (%) | 12.5 ± 5.3 | 10.6 ± 4.9 | 0.3 | 4.7 ± 3.3 | 6.4 ± 4.3 | 0.2 |
AV: atrio-ventricular; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; IVD: interventricular delay; SDI: systolic dyssynchrony index.
At follow-up, SR showed significantly better LV dimensions and function (Table 2). Absence of mitral regurgitation was still more frequent in SR. Dyssynchrony parameters improved in both groups, without showing significant differences between them.
According to our results, SR and non-SR had similar concentrations of NT-proBNP (3112.27 ± 3215.96 vs. 3215.96 ± 4716.6 ng/dL, p = 0.56), Gal-3 (20.0 ± 10.3 vs. 23.3 ± 11.3 ng/mL, p = 0.22), hsCRP (1.4 ± 0.8 vs. 1.8 ± 1.3 mg/mL, p = 0.57), IL-6 (37.6 ± 37.8 vs. 25.3 ± 18.0 pg/mL, p = 0.25) and TNFα (31.9 ± 61.1 vs. 20.5 ± 24.1 pg/mL, p = 0.92).
A tendency to a lower blood concentration of hsTnT was seen in SR compared to non-SR (0.04 ± 0.03 vs. 0.2 ± 0.5 ng/mL, p = 0.09). Concentrations of sCD40L were significantly higher in SR than in non-SR (6.9 ± 5.1 vs. 4.4 ± 3.3 ng/mL, p = 0.02).
To value a correlation between sCD40L and SR, we first analyzed a possible relationship between sCD40L and possible confounding factors. We could not find significant correlation between sCD40L and aspirin (r = -0.13, p = 0.94), clopidogrel (r = -0.11, p = 0.55), or aspirin and clopidogrel together (r = -0.09, p = 0.62). There were also no significant associations with anticoagulation therapy (r = -0.23, p = 0.18), arterial hypertension (r = 0.06; p = 0.7), statins (r = 0.04; p = 0.8) or ACE-inhibitors or ARBs (r = 0.2; p = 0.2). A moderate, direct correlation was found between SCD40L and SR (r = 0.39, p = 0.022), and with the improvement of LVESV at one year follow-up (r = 0.44, p = 0.02). The ROC curve for sCD40L and SR showed an AUC = 0.73 (95%CI: 0.56-0.89, p = 0.02) (Fig. 1).
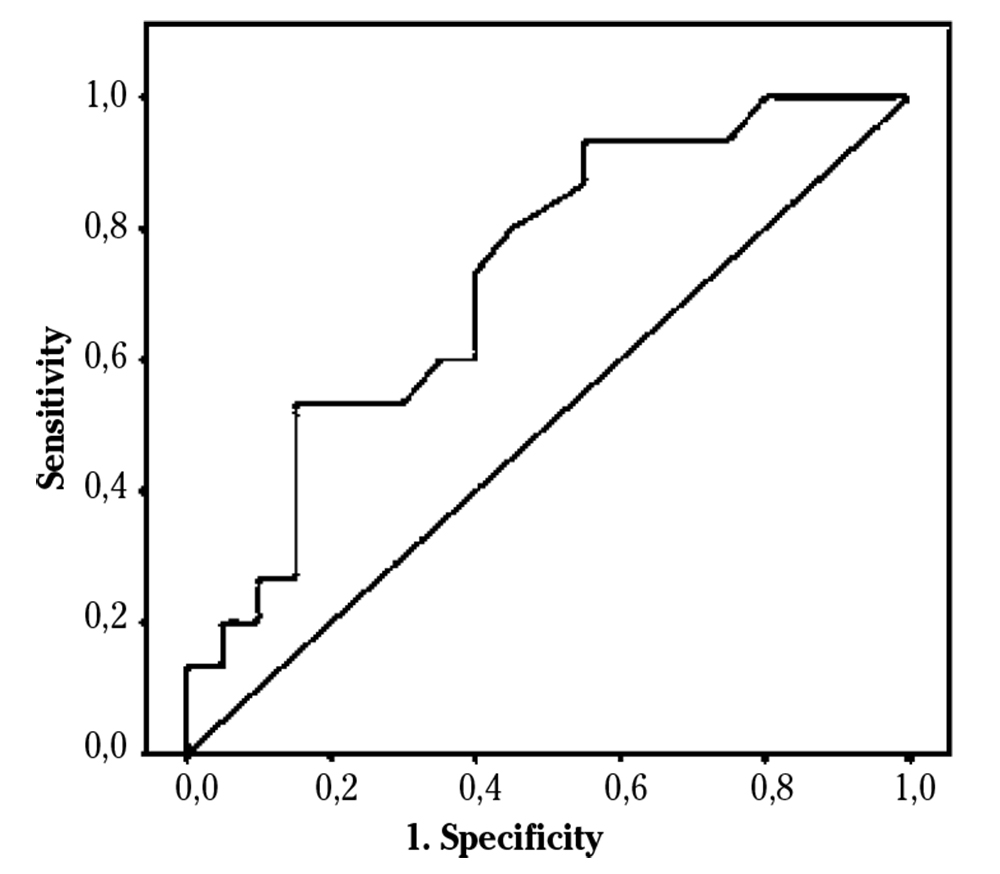
Figure 1. Receiving Operating Characteristic (ROC) curve relating sCD40L and super-response to cardiac resynchronization therapy.
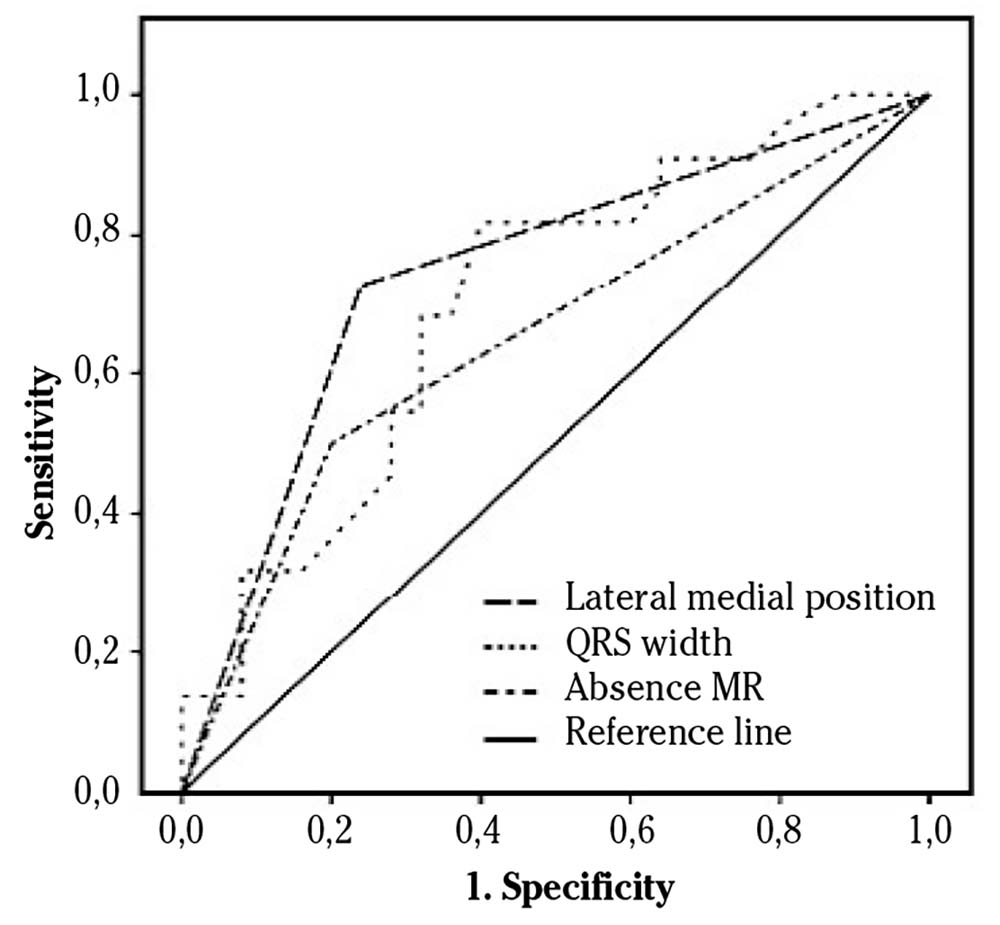
All significant values at baseline (female sex, ischemic cardiomyopathy, lateral-, inferior- and posterior infarctions, lateral medial position of LV lead, QRS width, absence of mitral regurgitation and sCD40L) and IVD were introduced into the univariate analysis. This first step showed significant differences in ischemic cardiomyopathy, inferior infarction, lateral medial position, QRS width, absence of mitral regurgitation and IVD. In the multivariate analysis, lateral medial position, mitral regurgitation and QRS width were independent predictors for SR (Table 3). The ROC curves showed an AUC of 0.74 for lateral medial position (CI95%: 0.59-0.89, p = 0.004), 0.70 for QRS width (95%CI: 0.55-0.85, p = 0.01) and 0.65 for absence of mitral regurgitation (95%CI: 0.5-0.8, p = 0.07) (Fig. 2).
Table 3. Univariate and multivariate analyses of the significant parameters for super-response to cardiac resynchronization therapy at baseline
| Univariate Analyses | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Sex (female) | 4.27 (0.97-18.65) | 0.054 | ||
| sCD40L | 1.17 (0.95-1.45) | 0.13 | ||
| Ischaemic cardiomyopathy | 0.09 (0.02-0.37) | 0.001 | 0.16 (0.006-4.53) | 0.28 |
| Lateral infarction | 0 | 0.99 | ||
| Inferior infarction | 0.09 (0.01-0.79) | 0.03 | 6.79 (0.09-467.25) | 0.37 |
| Posterior infarction | 0 | 0.99 | ||
| Lateral medial position of LV-lead | 6.53 (1.89-22.49) | 0.003 | 21.81(1.61-295.42) | 0.02 |
| QRS width | 1.04 (1.01-1.07) | 0.01 | 1.09 (1.02-1.18) | 0.01 |
| Absence MR | 3.5 (1.02-12.01) | 0.04 | 25.6 (1.38-476.08) | 0.03 |
| IVD | 1.04 (1.0-1.07) | 0.04 | 1.04 (0.97-1.16) | 0.31 |
OR: odds ratio; CI: confidence interval; MR: mitral regurgitation; IVD: interventricular delay.
Discussion
In this prospective study we hypothesized that novel cardiac biomarkers (IL-6, TNFα, sCD40L), as well as NT-proBNP, hsTnT, hsCRP and Gal-3 could predict SR to CRT. As a secondary endpoint, we looked for other clinical, electrocardiographic and echocardiographic predictors of SR to CRT. Our results show that the novel cardiac biomarker sCD40L correlates significantly with SR and the improvement of LVESV in patients with HF and CRT. Moreover, we demonstrate that QRS duration, absence of mitral regurgitation and location of the LV-lead in lateral medial position are predictive of SR with a probability around 70%.
CRT is an effective adjunctive therapy for the treatment of heart failure. It reduces death and events secondary to heart failure even in paucisymptomatic patients11. However, response to CRT is very variable. Even though 70% of patients seem to profit from the therapy, there are still 30% of them who do not show improvement or even worsen LVEF under CRT12. Those who improve the most are called SR. These patients have better survival and less appropriate ICD-shocks than non-SR2,3. However, SR have around 23% risk of ventricular arrhythmias at long-term follow-up, and therefore ICD implantation is justified13. During follow-up, one SR patient had an appropriate ICD-shock.
Nowadays, there is still a lack of consensus in the definition of SR. Therefore, it has been reported an incidence that varies from 9 to 47%13. CRT promotes LV reverse remodeling, and a reduction in LVESV≥ 15% is the most appropriate echocardiographic parameter to define it14. Therefore, we decided to define SR as the reduction of LVESV≥30%. Furthermore, this is one of the most used definitions in the literature7-9,12,15. Moreover, when comparing this definition of SR with other echocardiographic definitions, the long-term results are similar10.In our study, we detected 46% of SR. This high percentage could be attributed to the 60% of non-ischemic cardiomyopathy and not very dilated left ventricles. Finally, the follow-up period of 1 year allows us to evaluate SR at mid-term follow-up, which is associated with also better outcome at long-term follow-up2,3,9.
According to our results, sCD40L correlates directly with SR and also with the reduction of LVESV at the end of the study. Until the present study, this biomarker had never been studied as a potential predictor of SR in CRT. sCD40L is the soluble form of CD40L, and it is produced at platelet level. It participates in immune and inflammatory responses, as well as in platelet activation and aggregation16. The effect of antiplatelet therapy on sCD40L plasma concentration has been already studied. Kojok et al reported that ASA does not affect the concentration or function of sCD40L, demonstrating that the effect of sCD40L is independent of Tromboxane A216. On the other side, clopidogrel alone and double antiplatelet therapy seem to diminish the concentration of sCD40L in plasma, whereas female sex, high hsCRP and high hematocrit are predictive of higher concentrations17. On the other hand, arterial hypertension has been associated with an increase of sCD40L blood concentrations, especially in non-dipper hypertension18. On the contrary, statins, ACE-inhibitors and ARBs diminish blood concentrations of sCD40L19,20. In our study, there are no significant differences in antiplatelet therapy, arterial hypertension or cardiac medication among groups, and no correlations are found between these factors and sCD40L.
While evaluating the role of sCD40L in HF in the literature, we have found contrasted opinions. On one hand, some authors demonstrate that elevated concentrations of sCD40L in plasma are found in acute and chronic HF, and they correlate with disease severity21. It also acts as prognostic marker of disease progression and death22. Furthermore, it seems also to be increased in patients with ischemic cardiomyopathy who are at risk of reinfarction and death23. On the other hand, other authors do not find a correlation between sCD40L levels and disease severity or progression in stable HF, assuming that elevated sCD40L in this collective is related to comorbidities associated to HF23. The reason why sCD40L levels are increased in our SR is unclear. While our SR are more often women, non-SR have more ischemic cardiomyopathy. Moreover, no differences in antiplatelet therapy and oral anticoagulation have been found, and both groups show the same clinical and functional status at baseline. To our knowledge this is the first time that sCD40L is studied in CRT. Therefore, this is a pilot study and further investigation is necessary to clarify the usefulness of sCD40L.
In our study, longer QRS duration and LV lead in lateral medial position are predictive of SR at one year follow-up. Other factors, such as female sex, less ischemic cardiomyopathy and less lateral-, inferior- and posterior infarctions are significant in the SR group, even though they are not predictive in the multivariate model. These results are similar to a substudy of MADIT-CRT5, that identifies female sex, QRS ≥ 150 ms, LBBB, BMI < 30 kg/m2, left atrial diameter and absence of ischemic heart disease as predictors of SR. These similitudes occur even though our definition of SR differs from the MADIT substudy5 and our sample is much older and more heterogeneous, as we also include patients in NYHA III and IV, and patients with permanent RV-pacing and atrial fibrillation.
According to our echocardiographic results, SR do not differ to non-SR in cardiac function or morphology. Other studies5 show that a smaller left atrial volume index predicts SR. Unfortunately, we do not have this information in our study, but our SR had more often no mitral regurgitation at baseline. The less regurgitation preimplant, the better outcome24,25.On the other hand, mechanical dyssynchrony as predictor of CRT-response has been often investigated in small, non-randomized26 The first clinical trial in evaluating mechanical dyssynchrony was the PROSPECT study27, which failed to find a correlation between mechanical dyssynchrony and response to CRT. Analogically, we do not find significant differences in these parameters between SR and non-SR. Only IVD seems to have a tendency to significance between groups, being much longer in SR than in non-SR. Even though is statistically significant in the univariate analyses, it does not reach signification in the multivariate analyses.
Our study has been subject of some limitations. First, the small number of patients included and the short follow-up time makes it difficult to assess SR at long term follow-up. Furthermore, our cohort is very heterogeneous, which make the results more difficult to interpret. Nevertheless, our patients show the common variability in the daily clinical practice. Finally, our results have to be taken with caution due to the lack of consensus regarding the definition of SR.
In conclusion, sCD40L correlates with SR to CRT and with the reduction of LVESV at mid-term follow-up. Furthermore, QRS width, absence of mitral regurgitation at baseline and LV lead in lateral medial position are independent predictors of SR to CRT in our study.