Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.8 no.3 sep. 2005
| RESEARCH REVIEW | |||
|
| |||
| Microbiologically influenced corrosion: looking to the future
Summary. This review discusses the state-of-the-art of research into biocorrosion and the biofouling of metals and alloys of industrial usage. The key concepts needed to understand the main effects of microorganisms on metal decay, and current trends in monitoring and control strategies to mitigate the deleterious effects of biocorrosion and biofouling are also described. Several relevant cases of biocorrosion studied by our research group are provided as examples: (i) biocorrosion of aluminum and its alloys by fungal contaminants of jet fuels; (ii) sulfate-reducing bacteria (SRB)-induced corrosion of steel; (iii) biocorrosion and biofouling interactions in the marine environment; (iv) monitoring strategies for assessing biocorrosion in industrial water systems; (v) microbial inhibition of corrosion; (vi) use and limitations of electrochemical techniques for evaluating biocorrosion effects. Future prospects in the field are described with respect to the potential of innovative techniques in microscopy (environmental scanning electron microscopy, confocal scanning laser microscopy, atomic force microscopy), new spectroscopic techniques for the study of corrosion products and biofilms (energy dispersion X-ray analysis, X-ray photoelectron spectroscopy, electron microprobe analysis) and electrochemistry (electrochemical impedance spectroscopy, electrochemical noise analysis). [Int Microbiol 2005; 8(3):169-180] Key words: biocorrosion · bioelectrochemistry · biofilms · corrosion inhibition · biocides · sulfate-reducing bacteria | ||
| |||
Corrosión de origen microbiológico: mirando al futuro Resumen. Esta revisión describe la situación actual de la investigación en biocorrosión y bioensuciamiento (biofouling) de metales y de aleaciones de uso industrial. También se describen los conceptos clave necesarios para comprender los efectos principales de los microorganismos en el deterioro del metal, así como las tendencias actuales en las estrategias de supervisión y control para atenuar los efectos perjudiciales de la biocorrosión y del bioensuciamiento. Como ejemplos, se describen algunos casos destacados de biocorrosión estudiados por nuestro grupo de investigación: (i) biocorrosión del aluminio y de sus aleaciones por hongos contaminantes de los combustibles de motores de propulsión a chorro; (ii) corrosión del acero causado por bacterias sulfatorreductoras (SRB); (iii) interacciones de biocorrosión y bioensuciamiento en ambientes marinos; (iv) estrategias de supervisión para evaluar la biocorrosión en sistemas industriales del agua; v) inhibición microbiana de la corrosión; (vi) uso y limitaciones de las técnicas electroquímicas para evaluar los efectos de la biocorrosión. Se describen las perspectivas futuras en este campo en relación al potencial de las técnicas innovadoras en microscopia (microscopia electrónica de barrido ambiental, microscopia confocal laser de barrido, microscopia de fuerzas atómicas), de las nuevas técnicas espectroscópicas usadas para el estudio de la corrosión de productos y de los biofilmes (análisis mediante rayos-X de dispersión de energía, espectroscopia fotoelectrónica de rayos X, análisis mediante microsonda electrónica) y de la electroquímica (espectroscopia de impedancia electroquímica, análisis del ruido electroquímico). [Int Microbiol 2005; 8(3):169-180] Palabras clave: biocorrosión · bioelectroquímica · biofilmes · inhibición de la corrosion · biocidas · bacterias sulfatorreductoras | Corrosão de origem microbiológico: mirando ao futuro Resumo. Esta revisão descreve a situação atual da investigação em biocorrosão e bioensuciamiento (biofouling) de metais e de ligas de metais de uso industrial. Também se descrevem os conceitos chave necessários para entender os efeitos principais dos microorganismos na deterioração do metal, assim como as tendências atuais nas estratégias de supervisão e controle para atenuar os efeitos prejudiciais da biocorrosão e do bioincrustação. Como exemplos, se descrevem alguns casos destacados de biocorrosão estudados pelo nosso grupo de pesquisa: (i) biocorrosão do alumínio e de suas ligas de metais por fungos contaminantes dos combustíveis motores de propulsão a jorro; (ii) corrosão do aço causado por bactérias sulfatoreductoras (SRB); (iii) interações de biocorrosão e bioincrustação em ambientes marítimos; (iv) estratégias de supervisão para avaliar a biocorrosão em sistemas industriais da água; (v) inibição microbiana da corrosão; (vi) uso e limitações das técnicas electroquímicas para avaliar os efeitos da biocorrosão. Se descrevem as perspectivas futuras considerando o potencial das técnicas inovadoras em microscopia (microscopia eletrônica de varredura ambiental, microscopia confocal de varredura a laser, microscopia de forças atômicas), das novas técnicas espectroscópicas usadas para o estudo da corrosão de produtos e dos biofilmes (análise mediante raios-X de dispersão de energia, espectroscopia fotoelectrónica de raios X, análise mediante microsonda eletrônica) e da electroquímica (espectroscopia de impedancia electroquímica, análise do ruído electroquímico). [Int Microbiol 2005; 8(3):169-180] Palavras chave: biocorrosão · bioelectroquímica · biofilmes · inibição da corrosão · biocidas · bacterias sulfatoreductoras |
Introduction
Although the electrochemical nature of corrosion remains valid for microbiologically influenced corrosion (MIC), the participation of microorganisms in the process nonetheless induces several unique features, the most significant being the modification of the metal-solution interface by biofilm formation [61]. Biofilms affect interactions between metal surfaces and the environment, not only in biodeterioration processes such as MIC, but also in several biotechnological processes applied to materials recovery and handling [58]. Thus, the key to the alteration of conditions at a metal surface, and hence the enhancement or inhibition of corrosion is the formation of a biofilm [53]. This can be considered as a gel containing 95% or even more water and a matrix of exopolysaccharidic substances (EPS), in which microbial cells and inorganic detritus are suspended [16].
Biofilms formation on metals is the result of an accumulation process-not necessarily uniform in time or space [8]- that starts immediately after metal immersion in the aqueous environment. A thin film (approximately 20-80 nm thick), due to the deposition of inorganic ions and organic compounds of high relative molecular mass, is formed in a first stage. This initial film can alter the electrostatic charges and wettability of the metal surface, facilitating its further colonization by bacteria. In a short time (minutes or hours depending on the aqueous environment in which the metal is immersed), microbial growth and EPS production result in the development of a biofilm. This biofilm is a dynamic system, and the different transport processes and chemical reactions occurring at the biofouled interface will thus take place through the biofilm thickness [7].
Microbial colonization of metal surfaces drastically changes the classical concept of the electrical interface commonly used in inorganic corrosion: important changes in the type and concentration of ions, pH values, and oxidation-reduction potential are induced by the biofilm, altering the passive or active behavior of the metallic substratum and its corrosion products, as well as the electrochemical variables used for assessing corrosion rates [49]. Microorganisms influence corrosion by changing the electrochemical conditions at the metal-solution interface. These changes may have different effects, ranging from the induction of localized corrosion, through a change in the rate of general corrosion, to corrosion inhibition [52]. Any biological effect that either facilitates or impedes one of the anodic or cathodic reactions of the corrosion process, or that permanently separates anodic and cathodic sites, will increase corrosion. For instance, stimulation of the anodic reaction by acidic metabolites or the cathodic reaction by microbial production of a cathodic reactant such as hydrogen sulfide, the breakdown of protective films, or the increase in conductivity of the liquid environment enhances corrosion.
This review describes the current understanding of biocorrosion and biofouling of metals and alloys of industrial usage and draws on the experience accumulated during 30 years of research. The key concepts to understand the main effects of microorganisms on metal decay as well as current trends in monitoring and control strategies to mitigate biocorrosion and biofouling deleterious effects are discussed.
A new biologically conditioned interface
Simultaneous with the biological changes that lead to biofilm accumulation, a sequence of inorganic changes takes place at the metal surface immediately after its immersion in an aggressive aqueous medium. This sequence involves the process of metal dissolution and corrosion-product formation. Both biological and inorganic processes occur within the same time period, but in opposite directions at the metal-solution interface. Whereas corrosion and corrosion-product accumulation occur from the metal surface towards the solution, biofilm formation is the result of accumulation processes directed from the bulk towards the metal surface. Thus, a very active interaction between corrosion-product layers and biofilms can be expected. The consequent corrosion behavior of the metal varies depending on the degree of this reciprocal interaction, and a concept of a new biologically conditioned interface must be kept in mind [50]. The approach for a sound interpretation of any microbial corrosion case must then be interdisciplinary, and must include a thorough process analysis combined with well-defined microbiological and electrochemical methodologies [51].
Sulfate-reducing bacteria (SRB)-induced corrosion of carbon steel in a corrosive medium such as seawater is a good example of the complex interactions and effects taking place among inorganic corrosion products and biofilms in a biologically conditioned interface. In the presence of sulfur species (either biogenic or abiotic), carbon steel firstly develops a film of mackinawite that later changes through several chemical and electrochemical paths to more stable iron sulfides [54]. In all cases, iron sulfides are characterized by strong cathodic effects on the hydrogen reduction reaction, causing an indirect increase in the corrosion rate. Thus, the biocorrosion process will be related to the breakdown of steel passivity by corrosive metabolic products generated by SRB. Depending on both the sulfide concentration in the medium and the presence or absence of biofilms and EPS, the protective characteristics of the corrosion product may change. Biogenic layers of corrosion products can enhance protection by improving the adherence of the passive film to the metal, but can also increase corrosion by inducing heterogeneities at the metal surface [62].
The role of biofilms. The role of biofilms in enhancing corrosion in a biologically conditioned metal-solution interface can be diverse, and may proceed through simultaneous or successive mechanisms including:
(a) Alteration of the transport of chemical species from or towards the metal surface. The biofilm accumulates and forms a significant diffusion barrier for certain chemical species. For instance, a mature biofilm composed of microbial cells and their EPS prevents the diffusion of oxygen to cathodic areas and the diffusion of aggressive anions such as chloride to anodic sites. Outward diffusion of metabolites and corrosion products is also impeded [58]. (b) Facilitating the removal of protective films when the biofilm detaches. Copper-nickel alloys in seawater can be colonized by bacteria and other organisms after extended periods of exposure despite their perceived anti-fouling properties. Biofilm formation is here conditioned by the chemical nature and distribution of the inorganic passive layers [49] and by the elemental composition of the substratum [6]. After several months of exposure, bacteria can be found entrapped between layers of corrosion products and EPS in a layered structure. Biofilm detachment might facilitate the removal of inorganic passive layers, resulting in a patchy distribution of the biofilm. Stalked ciliates, observed sometimes as the predominant biofouling species, can facilitate passive layer detachment through adhesion effects developed at the fixation points of pseudopodia and assisted by water flow velocity. (c) Inducing differential aeration effects as a consequence of a patchy distribution of the biofilm. Non-uniform or patchy colonization by microbial biofilms results in the formation of differential aeration cells, where areas under respiring colonies are depleted of oxygen relative to surrounding non-colonized areas. These effects give rise to potential differences and, consequently, to corrosion currents. The areas under respiring colonies become anodic and there, metal dissolution occurs. Conversely, in the cathodic surrounding areas the counter-reaction of oxygen reduction takes place. (d) Changing oxidation-reduction conditions at the metal-solution interface. Diffusion of oxygen in oxic aqueous media is frequently impeded by the diffusion and reaction resulting from aerobic metabolites within the biofilm. Microelectrode measurements in a biofilm that accumulates in a flow [31] indicate that the dissolved oxygen can decrease to zero at a distance of only 180 µm from the metal surface. SRB, which need a reducing environment to grow, can proliferate at the bottom of biofilms despite a measurable dissolved oxygen concentration in the bulk water. Thus, redox conditions within the biofilm and at the biofouled metal surface are closely related to the respiration and metabolic activity of sessile microorganisms. (e) Altering the structure of inorganic passive layers and increasing their dissolution and removal from the metal surface. Some metabolic activities of microorganisms within the biofilm may markedly affect MIC. An example is the reducing capacity of ferric to ferrous ions inherent to certain bacteria [17]. In carbon steel surfaces immersed in a saline medium, localized attack preferentially occurred [15] beneath microbial colonies as a consequence of differential aeration between covered and uncovered areas, and the depassivation of the metal surface due to microbial reduction of insoluble ferric deposits into soluble ferrous compounds. Microbial consortia within the biofilm can enhance these effects, which accounts for an increase in concentration and variety of aggressive species.
Looking back to the twentieth century: the beginnings
Historical background and research trends. Even though the first reports on MIC go back to the turn of the twentieth century [14, 50], its rational interpretation only began to be rigorous in the mid-1960s. The only exception was the pioneer work of von Wolzogen Kuhr and van der Flugt, in 1934, which can be considered as the first attempt to interpret MIC electrochemically [66]. Until the 1960s, the few publications on the subject only reported practical cases, mainly those involving underground bacterial corrosion of iron pipes and structures [18, 42].
During the 1960s and the early 1970s, research on MIC was devoted either to objecting to or validating the anaerobic corrosion of iron by SRB as explained by the cathodic polarization theory. Some of the papers published during that period introduced the use of electrochemical techniques, such as polarization experiments, and corrosion potential versus time measurements, coupled with microbiological methods for assessing the effect of SRB on iron corrosion [4, 22]. Although experimental results were interpreted as a confirmation of the cathodic activity of SRB, corrosion rates obtained in the laboratory were substantially lower than those measured in the field.
One of the explanations for the difficulty in reaching an adequate understanding of MIC until the late 1970s was the lack of an appropriate transfer of knowledge among different areas, including metallurgy, electrochemistry, microbiology, and chemical engineering. Until then, most research in the field was carried out using laboratory measurements employing pure strains of microorganisms growing in complex culture media that were completely different from the real conditions generally found in practice. A key fact, related to the active interaction between microorganisms and metal surfaces like the settlement of biofilms, was ignored in MIC research [46].
Since the cathodic depolarization theory (CDT) of von Wolzogen Kuhr and van der Flugt was published in 1934 [66], MIC of carbon steel in anoxic environments involving the presence of SRB has been the focus of most of the research in the field. In this case of MIC, SRB were usually found in waterlogged clay soils with pH values near neutrality. In such environments, due to their neutral reaction and low oxygen content, abiotic corrosion of iron would be negligible or absent because neither of the two common cathodic reactants (protons or oxygen) would be available.
The basic idea of the CDT was that the removal of hydrogen from the cathodic area on the iron surface by the hydrogenases of the bacteria, coupled to the reduction of sulfate, to sulfide could account for the severe corrosion of iron in water-logged clay soils, as was found in practice. Thus, the corrosion reaction would be indirectly accelerated by depolarization of the cathodic reaction. The resulting corrosion products would thus be ferrous sulfide and ferrous hydroxide. The term "depolarization" was not used by the authors in a strict electrochemical sense; it merely indicated that there was an undefined change in the electrochemical behavior of iron. SRB, by using the adsorbed hydrogen in the sulfate reduction, would in fact increase the rate of corrosion by allowing the cathodic reaction to run at a faster rate, bypassing the recombination step of the adsorbed hydrogen atoms, which requires large activation energy [53]. In this way, the depolarization effect was used as an equivalent to a diminution of the activation energy for hydrogen removal, providing an alternative route of depolarization.
The indirect role of bacteria in accelerating corrosion, as proposed by the CDT, is very hard to accept in light of present knowledge on MIC. An indirect role of SRB in the anaerobic corrosion of iron was proposed by Costello [10], who suggested that cathodic polarization in SRB cultures may be due to dissolved hydrogen sulfide produced by the bacteria. Thus, the cathodic reaction could be expressed as follows: H2S + e = SH- + ½ H2
The role of bacteria in cathodic depolarization was minimized later by King and Miller, who attributed that effect to iron sulfide [24]. Therefore, the role of SRB would be limited to the removal of hydrogen atoms linked to ferrous sulfide crystals, whereas the iron sulfide lattice would act as cathodes for the hydrogen reaction. The depolarizing effect of ferrous sulfide on the hydrogen evolution reaction was soon confirmed [26].
In addition, the main weak point of the CDT was the lack of information on the role of sulfides in the stimulation of the anodic reaction. The complexity of biological environments involved with SRB activity makes it very difficult to assess any microbiological effect by means of electrochemical methods, because the chemical composition and pH of the medium are continuously varying due to microbial metabolism. Finally, there is another key fact generally not taken into account in the formulation of a mechanism to explain the anaerobic corrosion of iron: that, in practice, the iron surface is rarely free from other deposits (oxides, sulfides, hydroxides, and even biofilms). Under these circumstances, the interpretation of anaerobic corrosion of iron must necessarily consider the breakdown of passivity by metabolic products poured into the medium by SRB metabolic activity [54].
The 1980s: progress in understanding MIC
Relevant advances in MIC knowledge. During the 1980s, MIC became the focus of increasing attention from different research areas in response to the demands of a wide range of industries. Fortunately, an increasing intellectual and technical cross-fertilization of ideas between researchers from different disciplines, including microbiology, electrochemistry, and materials science, improved considerably the understanding of MIC. Special meetings or sessions on microbial aspects of corrosion were sponsored in the 1980s by the Institute of Petroleum, the Metal Society and the Biodeterioration Society in the United Kingdom, and by NACE International (the largest organization in the world committed to the study of corrosion), the American Society for Testing and Materials (ASTM), the American Welding Society (AWS) and the Electric Power Research Institute (EPRI), among others in the United States [55].
Also in the 1980s, several research groups carried out intensive work to elucidate MIC mechanisms, with the goal of better understanding the complex interactions occurring at biologically conditioned interfaces in biotic corrosion processes. The Center for Interfacial Microbial Process Engineering, in Bozeman, Montana, led by W.G. Characklis, had conducted, for the first time, an engineering process analysis of biofouling and MIC, which was described in a series of relevant publications and books [7,8,28]. Insight into MIC problems in the marine environment, such as crevice corrosion due to biofouling deposits as well as ennoblements effects due to biofilm interactions on the metal surface, were studied by Dexter and coworkers from the College of Marine Studies at the University of Delaware [12]. Other researchers that contributed actively to increased interest in MIC in the 1980s were Brenda Little and coworkers at the Naval Research Laboratory, David C. White and his group at the University of Tennessee, both in the USA, and in Europe, Allan Hamilton at the University of Aberdeen, Scotland and Robert G. E. Edyvean, at the University of Sheffield, and Christine Gaylarde at the City of London Polytechnic.
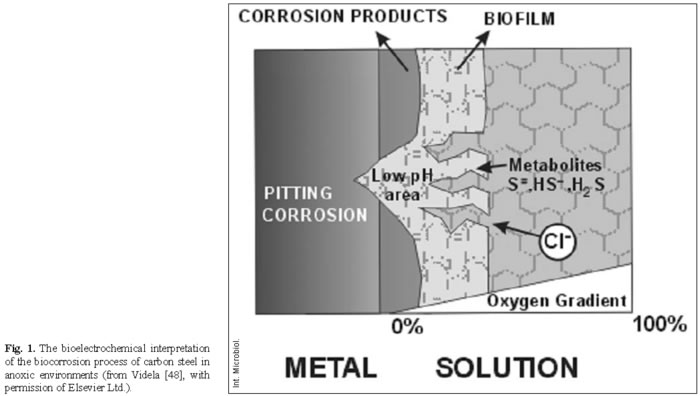
The main efforts of our research group at the Institute for Theoretical, Applied Physicochemical Research (INIFTA), University of La Plata, Argentina, during the 1980s focused on the electrochemical aspects of the anaerobic corrosion of iron. We conducted a series of laboratory experiments using alkaline and neutral buffered solutions as well as SRB cultures in saline media under well-defined experimental conditions [40,41,47). From the results of these studies, a bioelectrochemical interpretation of the MIC process of carbon steel in anoxic environments could be summarized as follows [52,53]: (a) biogenic sulfides affect localized corrosion of carbon steel in a manner similar to that of abiotic sulfides. The type and intensity of sulfide effects are closely related to the nature of the protective film already present on the metal surface. (b) In neutral media, sulfide ions lead to the formation of a poorly protective film of mackinawite. (c) The anodic breakdown of passivity is the first step in the corrosion reaction. Thus, the role of SRB is indirect, through the production of final metabolites (sulfides, bisulfides, hydrogen sulfide) or intermediate metabolites (thiosulfates, polythionates) that are corrosive to carbon steel. (d) Cathodic effects, such as cathodic depolarization, which have been attributed to either SRB hydrogenase or iron sulfide films, develop later than passivity breakdown during the corrosion process. (e) The corrosive action of biogenic sulfides can be enhanced by other corrosive ions already present in the medium (e.g. chlorides) and through the action of microbial consortia within biofilms on the metal surface. This bioelectrochemical approach is schematized in Fig. 1.
Industrial awareness of MIC. Microbial growth on metals and alloys of industrial usage affect the performance of a plant, structure, or component in different ways including: (a) decreased heat-transfer performance in heat exchangers, (b) induction of corrosion of the structural material, (c) increased pumping costs, (d) increased loading on marine structures and induction of corrosion fatigue or stress corrosion, (e) blockage of filter systems, and (f) spoilage of the final product.
A better understanding of the need to use proper sampling techniques regarding planktonic and sessile microorganisms complemented with the use of simple but selective culture media was achieved in the 1980s as a consequence of the industrial awareness of MIC. The most relevant innovative biological techniques used for assessing and monitoring MIC developed in that decade included ATP photometry [24], radiorespirometry techniques [19], fluorescence microscopy [37], immunological techniques [38], adenylyl sulfate (APS) reductase antibodies to detect SRB [46], monoclonal antibody probes [24], and hydrogenase assessment [3]. Several of these techniques were specifically applied in the oil industry to detect and certify MIC problems in water injection systems and offshore platforms. As an initiative of the Institute of Petroleum, in the UK, a conference was held in Aberdeen, Scotland, in April 1986, to discuss three major aspects of the deleterious effects of microbial growth: corrosion of steel and concrete, biofilm formation and macrofouling, and reservoir transformation and souring. Papers on these three relevant aspects of MIC were published later in a book [20].
Significant progress regarding the corrosion process was obtained during the 1980s in the electrochemical interpretation of MIC as a consequence of a better understanding of MIC complexity [12,35,51]. It was pointed out that the presence of complex deposits of corrosion products, microbial metabolites, and EPS may dramatically reduce the usefulness of several electrochemical techniques when assessing MIC. Microbial colonization of passive metals can drastically change their resistance to breakdown by altering locally the type and concentration of ions, pH values, oxygen gradients, and even inhibitor levels. These can result not only in important alterations in the electrochemical behavior of the metal, but may also change the results obtained by classical electrochemical techniques used for assessing inorganic corrosion [61].
A wide range of electrochemical techniques, such as corrosion and redox potentials measurements, Tafel and potentiodynamic polarization, linear polarization and electrical resistance probes, as well as several modern electrochemical techniques, including alternating current methods or electrochemical noise, have been critically reviewed by several authors in relation to their use in biocorrosion evaluation [12,51].
Monitoring MIC. Monitoring programs for MIC have focused mainly on the assessment of planktonic populations in water samples and generalized corrosion by using corrosion coupons or some kind of resistance or polarization resistance probes. However, some objections to this kind of monitoring probes have been made. One of them is that planktonic populations do not properly reflect the types and numbers of organisms living in biofilms and causing biodeterioration problems. In addition, the susceptibility of planktonic microorganisms to antimicrobial agents markedly differs from that of sessile microorganisms within the biofilm, mainly because of the protective action of their EPS. Monitoring methods must provide information on well-established biofilms such as those that develop in water systems. For corrosion assessment, the electrical resistance method, widely used in the industry, is only appropriate for indicating a change in the general corrosion rate, but the results are difficult to interpret in the presence of localized corrosion such as pitting, the most frequent form of attack found in MIC [53]. If biofilms or localized corrosion occur, the polarization resistance reveals that something is happening, but may not give an accurate measure of the corrosion rate. Only the use of any of these techniques jointly with other electrochemical methods or variables assessing localized corrosion hazard can provide valuable data for monitoring the deleterious effects of MIC and biofouling. Owing to the variables of dissimilar nature involved in biofouling and MIC, an effective monitoring program, either for the laboratory or for the field, must supply information on water quality, corrosive attack, sessile and planktonic bacteria populations, biofilms characteristics, and chemical composition of inorganic and biological deposits [53]
Sampling devices for monitoring MIC and biofilms can be simultaneously used to assess corrosion attack after the removal of biological and inorganic deposits, yielding wider and more useful information. Sampling devices may be either directly implanted or side-stream implanted. Metal coupons, generally made with the same structural material of the system, have a known surface area, which enables an accurate count of sessile bacteria per cm2 after biofilm detachment. Coupons are mounted in holding assemblies that are inserted in the pipework of the laboratory or industrial system.
The 1990s: evolution of the knowledge of MIC
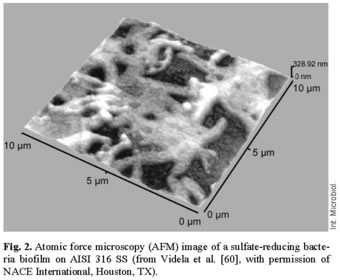
An updated overview of SRB-induced corrosion of iron. By the end of the 1990s and the beginning of the new century, several surface analysis techniques, electrochemical experiments, and microscopy observations had been employed to clarify the role of biotic and abiotic sulfide films in the corrosion behavior of steel in saline media [60, 63, 64]. Microbiological experiments were done under controlled laboratory conditions using a strain of Desulfovibrio alaskensis (D. alaskensis) isolated from a soured oil reservoir in Alaska and known for its ability to produce EPS [2]. Atomic force microscopy (AFM) was applied to image SRB biofilms (Fig. 2), current transients were measured to determine the electrochemical behavior of steel, and scanning electron microscopy (SEM) coupled with energy-dispersive analysis of X-rays (EDAX), as well as X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and electron microprobe analysis (EMPA) were carried out to examine the structure and composition of biotic and abiotic sulfide films on the surfaces of steel specimens.
Further studies developed in our laboratory [62,64] allowed us to conclude that SRB-influenced corrosion of steel is markedly affected by the nature and the structure of the sulfide films produced during metal dissolution. The environmental characteristics of the metal-biofilm/medium interface and its surroundings (pH, ionic composition, oxygen levels, EPS distribution) control the chemical and physical nature of corrosion product layers and may change their effects on the metal behavior from corrosive to protective. In marine environments, the impact of sulfur compounds on corrosion is enhanced by other aggressive anions, such as the widely distributed chlorides, already present in the medium.
The entrance of oxygen into anoxic environments accelerates corrosion rates, mainly through changes in the chemical nature of iron sulfides and elemental sulfur production. Both chemical species can provide additional cathodic reactants to the corrosion process, acting as electron carriers between the metal and the oxic interface within the biofilm. Differences between biotic and abiotic media containing identical levels of corrosive compounds (e.g. iron sulfides) can be attributed mainly to the presence of extracellular polymers and to heterogeneities at the metal surface due to the formation of a biofilm. In addition, the physicochemical variables of the corrosion product layers may change depending on the absence or presence of a biofilm, rendering these layers either protective or corrosive.
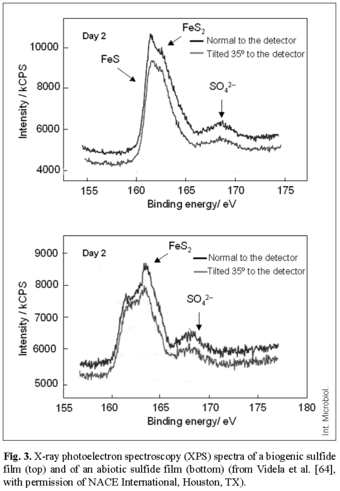
The main conclusions obtained from these studies were: (a) Both abiotic and biotic iron sulfide films are related to the formation of tubercles on the steel surface. For biotic solutions, however, FeS (mackinawite) predominates, whereas in abiotic media FeS2 (pyrite) is the major iron sulfide present (Fig. 3). (b) The structure of the outer crust of iron sulfide acts as a barrier for the diffusion of ions towards and from the pit cavity. (c) Biotic films adhere better to the metal surface, while abiotic films are flaky and loosely attached. (d) The previous history of the sulfide film may play a relevant role in the corrosion behavior of steel. The protective characteristics of the corrosion product may change depending on the sulfide concentration in the medium and on the presence or absence of biofilms and EPS. Biogenic layers of corrosion products can enhance protection by improving the adherence of the film to the metal, but can also increase corrosion, inducing the presence of heterogeneities at the metal surface.
Microbial inhibition of corrosion. Corrosion inhibition is the slowing down of the corrosion reaction and is usually performed by substances (corrosion inhibitors) that, when added in small amounts to a given environment, decrease the rate of attack by this environment on a metal. Microorganisms can change drastically the electrochemical conditions at the metal-solution interface. These changes can range from the induction or acceleration of corrosion to corrosion inhibition. Microbial effects that could enhance corrosion include the stimulation of the anodic reaction by acidic metabolites or the cathodic reaction by microbial production of a new alternative cathodic reactant (e.g. H2S), the microbial breakdown of protective films, and the increase in conductivity of the liquid environment. However, microbial effects causing corrosion inhibition have been hardly mentioned in the literature [52].
Microorganisms can contribute to corrosion inhibition by different mechanisms: neutralizing the action of corrosive substances present in the environment; forming protective films or stabilizing a pre-existing protective film on a metal; and inducing a decrease in the medium corrosiveness. General key features of microbial inhibition of corrosion can be summarized as follows [57]: MIC and its counter-process, microbial inhibition of corrosion, are rarely linked to a single mechanism of a single species of microorganisms. Either the corrosive or the inhibitory action of bacteria develop on biofilmed metal surfaces where complex biofilm/protective films occur. The main mechanisms of bacterial corrosion inhibition are always linked to a marked modification of the environmental conditions at the metal-solution interface due to biological activity. Microbial corrosion inhibition is frequently accomplished through: (i) a decrease in the cathodic rate by microbial consumption of a cathodic reactant (e.g. oxygen consumption by respiratory activity); (ii) decreasing the medium aggressiveness in restricted areas of the metal solution interface (e.g. by neutralizing acidity); and (iii) providing or stabilizing protective films on the metal (e.g. biofilm exopolymers with metal-binding capacity). In practical situations, the inhibitory action of bacteria can be reversed to a corrosive action within bacterial consortia structured in the biofilm thickness. Finally, a proper understanding of the identity and role of microbial contaminants in the specific environment of a metal surface may be exploited to induce corrosion inhibition by bacteria as a useful tool to prevent frequent MIC effects encountered in practice.
MIC prevention and control. An update. One of the classic concepts for maintaining an industrial system free of the deleterious effects of MIC is "to keep the system clean" [56]. Although in practice this is a very difficult task, several general methods (physical or chemical) can be used. Physical methods include flushing, which is perhaps the most simple, although of limited efficacy. A special case is the use of flushing supported by cleaners or jointly with chemical agents that induce biofilm detachment. Abrasive or non-abrasive sponge balls are frequently employed in industry. However, abrasive sponge balls can damage protective passive films, and non-abrasive sponge balls are not very effective with thick biofilms.
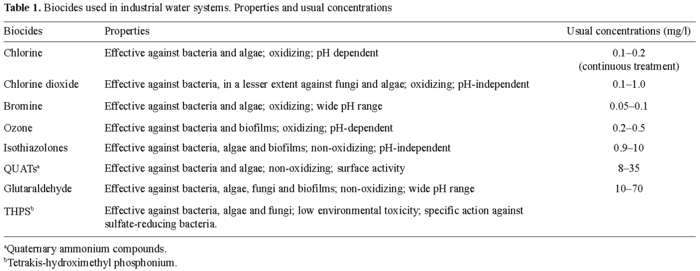
The most common chemical method for controlling biofouling in industrial water systems is the use of biocides. These can be either oxidizing or non-oxidizing toxicants. Chlorine, ozone and bromine are three typical oxidizing agents of industrial use. Non-oxidizing biocides are reported to be more effective than oxidizing biocides for overall control of algae, fungi, and bacteria as they are more persistent, and many of them are pH-independent. Combinations of oxidizing and non-oxidizing biocides or of two non-oxidizing biocides are often used to optimize the microbiological control of industrial water systems. Typical biocides of the second type are formaldehyde, glutaraldehyde, isothiazolones, and quaternary ammonia compounds (Table 1).
Increasing legislative requirements and the necessity for greater environmental acceptability have contributed to restricting the use of some traditional biocides and to developing either new compounds or carefully selected blends of existing biocides. Taking into account environmental concerns, the use of ozone for different types of industrial water systems presents several advantages with respect to other biocides [39]. The unique combination of the high toxicity of ozone during treatment and no toxicant discharge could make ozone the biocide of choice for the present decade, provided an appropriate balance between positive effects and costs is reached. Several publications from our laboratory on the biocidal action of ozone on sessile and planktonic bacteria, its mechanisms of disinfection, and the optimization of its use can be found in the literature [45,65].
Among the most promising non-oxidizing biocides, THPS (tetrakis-hydroximethyl phosphonium sulfate) is a new compound with wide-spectrum efficiency on bacteria, fungi, and algae. It is being widely used in the oil industry due to its ability to dissolve the ferrous sulfide. Its main advantage is its low environmental toxicity.
The twenty-first century: looking to the future
New tools for MIC studies. Significant improvements in analytical, microbiological, electrochemical, and microscopy techniques and instrumentation have allowed the development of new methods for laboratory and field assessment of MIC in industrial systems. Chemical analysis within the biofilm by means of microsensors is one of the most exciting advances in instrumentation [29]. Biofilm systems have been considered to be diffusion-limited [8]. As a consequence, chemical conditions at the surface and within biofilms can vary dramatically over a distance of a few micrometers. Thus, the information obtained from bulk water analysis is of limited value and must be closely analyzed before any conclusions about the behavior of the metal-solution interface can be drawn. Direct measurements inside biofilms are restricted by the small thickness of the biofilm, the diffusion limitation of concentration profiles across the biofilm, and the heterogeneous nature of the biofilm itself. The latter aspect is especially important not only in relation to microbial coverage of the surface but also with respect to MIC. An example of microsensor technology applied to evaluate vertical profiles of chemical species in biofilm systems has been reported [29]. Equally important tools for the study of biofilm structure are the fiber-optic microprobe or optrode, used for locating the biofilm-bulk water interface, and the mapping of electric fields [32] by means of the scanning vibrating microprobe (SVM).
Advanced microbiological techniques, such as DNA probes, have been applied recently to MIC and biofouling research [27]. Their joint application with microbiological field measurements can be highly useful for monitoring MIC. Environmental scanning electron microscope (ESEM), confocal laser scanning microscopy (CLSM) and atomic force microscopy (AFM) allow biofilm observation in real time and without introducing distortion of the samples. There is an increasing number of reports of these innovative technologies in the recent MIC literature [43,44] A combination of CSLM and microelectrode techniques allowed correlation of oxygen concentration profiles with biofilm structure [11,32]. CLSM facilitates the visualization of biofilm structures by eliminating the interference arising from out-of-focus objects [5]. Observations performed under flow conditions and using physiologically active biofilms have provided information to construct a new conceptual model of biofilm structure.
Recent advances in research. Molecular techniques involving the bacterial DNA and RNA are among the most exciting and promising techniques in MIC research. They offer the potential to: (a) identify dominant bacteria in a given ecological system without the serious limitations of standard viable counting techniques; (b) calculate the proportion of MIC-contributing bacteria in the total population; (c) identify bacteria that are susceptible or resistant to antimicrobials; (d) assess the changes in the overall population caused either by the use of biocides or nutrient modifications; and (e) achieve a more reliable sampling, not affected by time or transport factors.
Although molecular biology methods were used at the beginning of the 1990s [67], active research has developed in this field mainly in relation to its use in the oil industry [23,68]. A large bank of 16S rRNA sequence data on microorganisms is currently available due to the progress of microbial ecology and advances in molecular techniques. The classical sequence of techniques involves: DNA extraction, the use of polymerase chain reaction (PCR) to amplify copies of a particular gene in the sample, examination of the PCR products by a community fingerprint technique, such as denaturing gradient gel electrophoresis (DGGE), and the cloning of a particular DNA which is later sequenced and compared against DNA information contained in the data base. Although this method is qualitative, the use of more advanced systems, including quantitative PCR (QPCR), provides quantitative measures of the distribution and expression of target DNA or RNA. The use of DNA probes (small pieces of complementary DNA that bind to a specific part of DNA in the target microorganism) to identify and even quantify non-cultivated microorganisms in environmental samples is gaining in popularity. Reverse sample genome probing (RSGP) is a specific method used to analyze the microbiological diversity in a given ecosystem and it is particularly useful to monitor the effects of a biocide program [36].
The use of biocompetitive exclusion (BE) strategies is increasing in attempts by the oil industry to inhibit SRB-mediated reservoir souring and MIC. The addition of nutrients that stimulate the growth of competing bacterial populations (namely nitrate-reducing bacteria, NRB) effectively displace SRB from a microbial community by BE. Thus, the addition of nitrate can induce a shift in the dominant population from SRB to NRB. The use of nitrate to control SRB and hydrogen sulfide production in oil fields is nowadays a proven biotechnology whose effectiveness has been shown both in the laboratory and in several field studies [13]. Although the microbiological basis is well-understood, it is still not clear whether heterotrophic or autotrophic NRB play the most important role. From the present knowledge, nitrate amendment (in some cases phosphates or organic acid) stimulates NRB in oil-field waters and it therefore appears that an inoculum of NRB is not necessary.
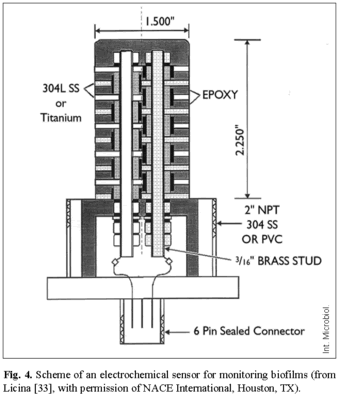
This replacement of generally toxic biocides using an environmentally friendly approach has proven to be successful in the oil fields of the North Sea [13,36], not only to control sulfide levels but also to reduce MIC effects. Corrosion sensors and electrochemically based corrosion assessment technology have received particular attention as effective and reliable tools to detect real time MIC effects. As an example, an electrochemical sensor for monitoring biofilms on metallic surfaces in real time was recently presented [33]. The system provides an immediate indication of the condition of biological activity on probe surfaces and it is a powerful tool to optimize biocide treatment (Fig. 4). This type of probe consists of a stack of nominally identical stainless steel discs (or other passive metals, such as titanium) that are configured as a right circular cylinder, the electrodes being electrically isolated from each other and from the body of the probe [34]. One set of discs is polarized negative to the other for a short period of time each day. The externally applied potential creates different local conditions on the stainless steel electrodes and provides a current that can be readily measured. If the applied current is tracked on a daily basis, a significant increase in that current provides a method to detect the onset of biofilm formation. The difference in the magnitude of the applied current from the baseline (the applied current in the absence of biofilm) provides a measure of biofilm activity. This kind of device has been successfully used jointly with integrated data acquisition and data analysis capabilities for monitoring biofilm activities on metallic surfaces to optimize biocide additions in a plant.
General remarks
MIC is rarely linked to a single mechanism or to a single species of microorganisms. Biofilms mediate interactions between metal surfaces and the liquid environment, leading to major modifications of the metal-solution interface by drastically changing the types and concentrations of ions, pH, and oxygen levels. As a consequence of these changes, the electrochemical behavior of the metal can be modified from active to passive and even a microbial inhibition of corrosion can be reached. The use of new analytical tools, molecular biology methods, and innovative electrochemical devices is increasing in MIC research. The new trends in research should focus on bioelectrochemical phenomena at the metal surface, the development of real-time monitoring devices, and methods to control microbial deleterious effects through environmentally friendly approaches such as BE strategies.
Acknowledgements. H.A. Videla acknowledges the financial support of the Agencia de Promoción Científica y Tecnológica of Argentina through the project PICT/99 6782 (Biodeterioration of materials).
References
1. Beech IB (1996). The potential use of atomic force microscopy for studying corrosion of metals in the presence of bacterial biofilms-an overview. Int Biodeter Biodegr 37:141-149 [ Links ]
2. Beech IB, Zinkevich V, Tapper R, Gubner R (1998) The direct involvement of extracellular compounds from a marine sulphate-reducing bacterium in deterioration of steel. Geomicrobiol J 15:121-134 [ Links ]
3. Boivin J (1990). The influence of enzyme systems on MIC. Paper No. 128, Corrosion 90, NACE International, Houston, TX [ Links ]
4. Booth GH, Tiller AK (1962) Polarization studies of mild steel in cultures of sulphate-reducing bacteria. Part 2. Thermophilic organisms. Transact Faraday Soc 58:110-115 [ Links ]
5. Caldwell DE, Korber DR, Lawrence JR (1992) Confocal laser microscopy and digital image analysis in microbial ecology. Adv Microbial Ecol 12:1-67 [ Links ]
6. Chamberlain AHL, Garner BJ (1988) The influence of iron content on the biofouling resistance of 90/10 copper-nickel alloys. Biofouling 1:79-96 [ Links ]
7. Characklis WC (1981). Fouling biofilm development: a process development. Biotechnol Bioeng 23:1923-1960 [Published online 2004; DOI 10.1002/bit.260230902] [ Links ]
8. Characklis WC, Marshall KC (eds) (1990) Biofilms, John Wiley & Sons, New York, 796 pp [ Links ]
9. Characklis WG, Wilderer PA (eds) (1989) Structure and function of biofilms, John Wiley & Sons, Chichester, UK, 387 pp [ Links ]
10. Costello, J.A., 1974. Cathodic depolarization by sulphate-reducing bacteria, South African J Science 70:202-204 [ Links ]
11. Costerton JW (1994) Structure of biofilms. In: Geesey GG, Lewandowski Z, Flemming HC (eds) Biofouling and biocorrosion in industrial water systems, Lewis Publishers, Boca Raton, FL, pp 1-14 [ Links ]
12. Dexter SC, Duquette DJ, Siebert OW, Videla HA (1991) Use and limitations of electrochemical techniques for investigating microbiological corrosion. Corrosion 47:308-318 [ Links ]
13. Eckford RE, Fedorak PM (2004). Using nitrate to control microbially-produced hydrogen sulfide in oil field waters. In: Vazquez-Duhalt R, Quintero-Ramírez R (eds) Petroleum biotechnology. Developments and perspectives, Elsevier, Amsterdam, The Netherlands, pp 307-340 [ Links ]
14. Gaines RH (1910) Bacterial activity as a corrosion induced in the soil. J Engineer Ind Chem 2:128-130 [ Links ]
15. Gaylarde CC, Videla HA (1987). Localized corrosion induced by a marine Vibrio. Int Biodegr 23:91-104 [ Links ]
16. Geesey GG (1982) Microbial exopolymers: ecological and economic considerations. ASM News 48:9-14 [ Links ]
17. Ghiorse WC (1988) Microbial reduction of manganese and iron. In: A.J.B. Zehnder (ed) Biology of anaerobic microorganisms, John Wiley, New York, pp 305-331 [ Links ]
18. Hadley RF (1948) Corrosion by microorganisms in aqueous and soil environments. In: Uhlig HH (ed) Corrosion handbook. John Wiley, New York, pp 466-481 [ Links ]
19. Hamilton WA (1985) Sulfate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol 39:195-217 [ Links ]
20. Hill EC, Shennan JL, Watkinson RJ (1987) Microbial problems in the offshore oil industry. The Institute of Petroleum - John Wiley & Sons, Chichester, UK, 257 pp [ Links ]
21. Horvath J, Solti M (1959) Mechanisms of anaerobic microbiological corrosion of metals in soil. Wekstoffe und Korrosion 10:624-630 [ Links ]
22. Iverson WP (1966) Direct evidence for the cathodic depolarization theory of bacterial corrosion. Science 151:986-988 [ Links ]
23. Jan-Roblero J, Romero JM, Amaya M, Le Borgne S (2004) Phylogenetic characterization of a corrosive consortium isolated from a sour gas pipeline, Appl Microbiol Biotechnol 64:862-867 [ Links ]
24. King RA (1995) Monitoring techniques for biological induced corrosion. In: Gaylarde CC, Videla HA (eds) Bioextraction and biodeterioration of metals. Cambridge University Press, Cambridge, UK, pp 271-305 [ Links ]
25. King RA, Miller JDA (1971) Corrosion by sulphate-reducing bacteria, Nature 233:491-492 [ Links ]
26. King RA, Miller JDA, Smith JS (1973) Corrosion of mild steel by iron sulfides. Brit Corrosion J 8:137-142 [ Links ]
27. Le Borgne S, Jan J, Romero JM, Amaya M (2002). Impact of molecular biology techniques on the detection and characterization of microorganisms and biofilms involved in MIC. Paper No. 02461, NACE International, Houston, TX [ Links ]
28. Lee W, Lewandowski Z, Nielsen PH, Hamilton WA (1995) Role of sulphate-reducing bacteria in corrosion of mild steel: a review. Biofouling 8:165-194 [ Links ]
29. Lewandowski Z (1994) Dissolved oxygen gradients near microbially colonized surfaces. In: Geesey GG, Lewandowski Z, Flemming HC (eds) Biofouling and biocorrosion in industrial water systems, Lewis Publishers, Boca Raton, FL, pp 175-188 [ Links ]
30. Lewandowski Z., Funk T, Roe F, Little B (1994). Spatial distribution of pH at mild steel surfaces using an iridium oxide microelectrode. In: Kearns JR, Little BJ (eds) Microbiologically influenced corrosion testing, ASTM Publications STP 1232, Philadelphia, PA, pp 61-69 [ Links ]
31. Lewandowski Z, Lee WC, Characklis WG, Little BJ (1988) Dissolved oxygen and pH microelectrode measurements at water-immersed metal surfaces. Paper No. 93, Corrosion 88, NACE International, Houston, TX [ Links ]
32. Lewandowski Z, Roe F, Funk T, Chen D (1993) Proc. NSF-CONICET Workshop, Biocorrosion and Biofouling: Metal/Microbe Interactions, Buckman Laboratories International, Memphis, TN, pp 52-61 [ Links ]
33. Licina GJ (2001) Monitoring biofilms on metallic surfaces in real time. Paper No. 01442, Corrosion 2001, NACE International, Houston, TX [ Links ]
34. Licina GJ (2004) Optimizing biocide additions via real time monitoring of biofilms. Paper No. 04582, Corrosion 2004, NACE International, Houston, TX [ Links ]
35. Mansfeld F, Little BJ (1990). The application of electrochemical techniques for the study of MIC. A critical review. Paper No. 108, Corrosion 90, NACE, Houston, TX [ Links ]
36. Maxwell S, Devine C, Rooney F, Spark I (2004) Monitoring and control of bacterial biofilms in oilfield water handling systems. Paper No. 04752, Corrosion 2004, NACE International, Houston, TX [ Links ]
37. Paul JH (1982) Use of Hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol 43:934-944 [ Links ]
38. Pope DH, Zintel TP (1988) Methods for the investigation of under-deposit microbiologically influenced corrosion. Paper No. 249, Corrosion 88, NACE International, Houston, TX [ Links ]
39. Rice RG, Wilkes JF (1991) Fundamental aspects of ozone chemistry in recirculating cooling water systems. Paper No. 2005, Corrosion 91, NACE International, Houston, TX [ Links ]
40. Salvarezza RC, Videla HA, Arvía AJ (1982) The electrodissolution and passivation of mild steel in alkaline sulfide solutions, Corrosion Sci 22:815-829 [ Links ]
41. Salvarezza RC, Videla HA, Arvía AJ (1983) The electrochemical behavior of mild steel in phosphate-borate-sulfide solutions, Corrosion Sci 23:717-732 [ Links ]
42. Starkey RL, Wight KM (1945) Anaerobic corrosion of iron in soil. American Gas Association, New York [ Links ]
43. Steele A, Goddard D, Beech IB (1990) The use of atomic force microscopy in study of the biodeterioration of stainless steel in the presence of bacterial biofilms, Int Biodeter Biodegr 34:35-46 [ Links ]
44. Steele A, Beech IB, Goddard DT (1995). A guide to visualization of single bacterial cells, biofilms and corrosion damage of stainless steel using the technique of atomic force microscopy. In: Angel P, Borestein SW, Buchanan RA et al. (eds) Proc. 1995 International Conference on Microbially influenced corrosion, American Welding Society-NACE International, Houston, TX [ Links ]
45. Strittmatter RJ, Bo Yang, Johnson DA (1992) Application of ozone in cooling water systems. Paper No. 347, Corrosion 92, NACE International, Houston, TX. [ Links ]
46. Tatnall RE, Stanton KM, Ebersole RC (1988). Methods of testing for the presence of sulphate-reducing bacteria. Paper No. 88, Corrosion 88, NACE International, Houston, TX [ Links ]
47. Videla HA (1986) Corrosion of mild steel induced by sulphate-reducing bacteria-a study of passivity breakdown by biogenic sulfides. In: Dexter SC (ed) Biologically induced corrosion. NACE-8, Houston, TX, pp 162-171 [ Links ]
48. Videla HA (1988) Electrochemical interpretation of the role of microorganisms in corrosion. In: Houghton DR, Smith RN, Eggins HOW (eds) Biodeterioration 7. Elsevier Applied Science, London, England, pp 359-371 [ Links ]
49. Videla HA (1989) Metal dissolution/redox in biofilms. In: Characklis WG, Wilderer PA (eds) Structure and function of biofilms. John Wiley, Chichester, UK, pp 301-320 [ Links ]
50. Videla HA (1991) Microbially induced corrosion: an updated overview. In: Rossmoore HW (ed) Biodeterioration and biodegradation 8. Elsevier Applied Science, London, pp 63-88 [ Links ]
51. Videla HA (1995) Electrochemical aspects of biocorrosion. In: Gaylarde CC, Videla HA (eds) Bioextraction and biodeterioration of metals. Cambridge University Press, Cambridge, UK, pp 85-127 [ Links ]
52. Videla HA (1996) Corrosion inhibition in the presence of microbial corrosion. Paper No. 223, Corrosion 96, NACE International, Houston, TX [ Links ]
53. Videla HA (1996) Manual of biocorrosion. CRC Press, Boca Raton, FL, 273 pp [ Links ]
54. Videla HA (2000) An overview of mechanisms by which sulphate-reducing bacteria influence corrosion of steel in marine environments. Biofouling 15:37-41 [ Links ]
55. Videla HA (2001) Microbially induced corrosion: an updated overview. Int Biodeter Biodegr (Special Millennium Issue) 48:176-201 [ Links ]
56. Videla HA (2002) Prevention and control of biocorrosion. Int Biodeter Biodegr 49:259-270 [ Links ]
57. Videla HA (2003) Biocorrosion and biofouling of metals and alloys of industrial usage. Present state of the art at the beginning of the new millennium. Revista de Metalurgia, Madrid, Vol. Extr. 256-264 [ Links ]
58. Videla HA, Characklis WC (1992) Biofouling and microbial influenced corrosion. Int Biodeter Biodegr 29:195-212 [ Links ]
59. Videla HA, de Mele MFL, Brankevich GJ (1989) Biofouling and corrosion of stainless steel and 70/30 copper-nickel samples after several weeks of immersion in seawater. Paper No. 291, Corrosion 89, NACE International, Houston, TX [ Links ]
60. Videla HA, Edyvean RG, Swords CL, de Mele MFL, Beech IB (1999) Comparative study of the corrosion product films formed in biotic and abiotic media. Paper 163, Corrosion 99, NACE International, Houston, TX [ Links ]
61. Videla HA, Herrera LK (2004) Biocorrosion. In: Vazquez-Duhalt R, Quintero-Ramírez R (eds), Petroleum biotechnology. Developments and perspectives. Elsevier, Amsterdam, The Netherlands, pp 193-218 [ Links ]
62. Videla HA, Herrera LK, Edyvean RG (2005) An updated overview of SRB induced corrosion and protection of carbon steel. Paper No. 05488, Corrosion 2005, NACE International, Houston, TX [ Links ]
63. Videla HA, Swords CL, de Mele MFL, Edyvean RG, Watkins P, Beech IB (1998) The role of iron in SRB influenced corrosion of mild steel. Paper No. 289, Corrosion 98, NACE International, Houston, TX [ Links ]
64. Videla HA, Swords CL, Edyvean RG (2002) Corrosion products and biofilm interaction in the SRB influenced corrosion of steel. Paper 02557, Corrosion 2002, NACE International, Houston, TX [ Links ]
65. Videla HA, Viera MR, Guiamet PS, de Mele MFL, Staibano Alais JC (1995) Biocide activity of ozone on sessile and planktonic bacteria: effects of corrosion behaviour of cooling water systems structural materials. In: Angel P, Borestein SW, Buchanan RA et al (eds) (Proc. 1995 International Conference on Microbially Influenced Corrosion, American Welding Society-NACE International, Houston, TX, 62-1 [ Links ]
66. von Wolzogen Kuhr CAH, van der Flugt LS (1934) De grafiteering van gietijzer als electrobiochemisch proces in anaerobe gronden. Water (den Haad) 18:147-165. [English translation published in 1961: Corrosion 17:293-299] [ Links ]
67. Westlake DWS,Voordouw G, Jack TR (1993) Use of nucleic acid probes in assessing the community structure of sulphate-reducing bacteria in Western Canadian oil field fluids, Proceedings 12th International Corrosion Congress, NACE International, Houston, TX, 3794-3802 [ Links ]
68. Zhu X, Kilbane II JJ (2004) Molecular tools in microbial corrosion. In: Vazquez-Duhalt R, Quintero-Ramírez R (eds) Petroleum biotechnology. Developments and perspectives. Elsevier, Amsterdam, The Netherlands, pp 219-232 [ Links ]