Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Enfermería Global
versión On-line ISSN 1695-6141
Enferm. glob. vol.18 no.55 Murcia jul. 2019 Epub 21-Oct-2019
https://dx.doi.org/10.6018/eglobal.18.3.316971
Originals
Sociodemographic and clinical characterization of patients with chronic hepatitis C
1Nurse by the Nursing Department of the Federal University of Rio Grande do Norte. Natal, RN, Brazil.
2Nursing Academic. Department of Nursing. Federal University of Rio Grande do Norte. Natal, RN, Brazil.
3Nurse. Master's Degree in Nursing by the Graduate Program in Nursing, Federal University of Rio Grande do Norte. Natal, RN, Brazil. vanessabarreto10@gmail.com
4Nurse. PhD in Nursing from the Federal University of Ceará. Adjunct Professor, Department of Nursing, Federal University of Rio Grande do Norte. Natal, RN, Brazil.
Objective
To describe the sociodemographic and clinical characterization of patients with chronic hepatitis C followed at the outpatient clinic of a reference hospital in infectology.
Method
A cross-sectional, descriptive, quantitative study with chronic hepatitis C patients attended at a referral hospital during November 2015 to April 2016 with a sample of 47 users.
Results
The participants were male (76.6%), 57 years old (57.5%), brown (38.3%), married (55.3%), (61.7%), with a discovery time of up to 6 years (68.1%), not knowing the form of contamination (57.5%), immunized against hepatitis B (65.9%), undergoing drug therapy (85.1%) with Ribavirin (55.6%); And 70.2% had adverse effects.
Conclusion
Sociodemographic and clinical characterization assist the clinical practice of the multiprofessional team with patients with chronic hepatitis C.
Keywords: Hepatitis C, Chronic; epidemiology; Continuity of Patient Care
INTRODUCTION
Infection by the hepatitis C virus (HCV) is considered one of the most serious causes of liver disease. According to the World Health Organization (WHO), an estimated 130 million to 150 million people worldwide are infected with HCV, of which 71 million have a chronic infection and approximately 399,000 people die each year from hepatitis C, mainly due to cirrhosis and hepatocellular carcinoma. Therefore, considering it an emerging disease1,2,3.
According to the Epidemiological Bulletin of Viral Hepatitis in Brazil, approximately 152,712 confirmed cases of hepatitis C were reported in the country between 1999 and 2015. Regarding the mortality coefficient due to hepatitis C as the underlying cause, it is assumed that for Brazil there is a stabilization trend from 20074.
The spread through HCV occurs initially in the blood and is directed to the liver. The virus, in turn, invades the hepatocyte, its capsule is ruptured intracellularly and replicated, thus causing cell death and hepatic inflammation. However, since there are several genotypes for HCV, the organism cannot create an immune response against the virus, so the inflammation internalizes in the liver, in structures that have a low regeneration capacity, clinically advancing from fibrosis I or II to III and IV characterized by cirrhosis and hepatocarcinoma5.
In relation to the clinical evolution, a more reserved prognosis is expected than the other types of hepatitis, due to its greater potential of chronification, being able to reach 85% of the cases. It is noteworthy that parts of the people affected by the virus can develop asymptomatically with subclinical or constitutional symptoms, such as nausea, asthenia, inappetence, and jaundice, which is present only in 18 to 26% of cases. This implies difficulty in identifying the HCV infection, and culminates in a late diagnosis of hepatitis C, which is most often performed in the chronic stage6.
Regarding treatment, the drugs act as a mechanism of direct action on the DNA and RNA of the virus, inhibiting its replication. Thus, at the beginning of 2016, a new protocol of therapeutic guidelines for hepatitis C and co-infections was introduced, replacing the previous one that brought Ribavirin and Interferon as main drugs, but presenting numerous adverse effects7,8. Therefore, the new drug regimen encompasses drugs administered orally, with shorter treatment times, and a decrease in side effects, which facilitates the adherence of the users and the follow-up of the professionals8,9.
It is worth pointing out that in order to achieve a sustained virological response, the patient needs an outpatient follow-up that includes follow-up of the drug therapy, with a view to the dimensions of the adherence process, attendance of scheduled appointments, besides a multiprofessional approach8.
Considering that hepatitis C is considered a disease of great epidemiological importance worldwide, it is necessary to know the sociodemographic and clinical profile of patients with hepatitis C in order to provide the health team with knowledge about the style of life, as well as, to act in the control of factors that can potentiate the progression of the disease.
The aim of this study was to describe the sociodemographic and clinical characterization of patients with chronic hepatitis C, who were followed at the outpatient clinic of a reference hospital in infectious diseases.
METHODOLOGY
This is a cross-sectional, descriptive, quantitative study developed at the Giselda Trigueiro Hospital (HGT), a reference in the State of Rio Grande do Norte (RN) for infectious diseases, toxology and immunobiology, which takes care of patients through the Unified Health System (SUS).
The research population consisted of chronic hepatitis C users, assisted by professionals from the outpatient clinic. As inclusion criteria, the following criteria were chosen: age equal to or greater than 18 years, both sexes and follow-up during the data collection period of this study.
The sampling process was carried out for convenience, as the follow-up consultations were carried out after their prior scheduling. Patients were invited individually to go to a separate room while waiting for their consultation, explaining the purpose of the research, the confidentiality of the data informed by research ethics and asked if they wanted to collaborate with the research. Thus, the final sample consisted of the 47 users who passed the outpatient clinic and met inclusion criteria from November 2015 to April 2016.
The instrument used for data collection was developed based on the clinical protocol and therapeutic guidelines for viral hepatitis C and coinfections10. It consisted of a structured interview divided into two stages: the first containing 10 sociodemographic items: gender, age, race, marital status, educational level, occupation, family income, city of origin; and the second with 14 questions about the treatment of hepatitis C from the patients' perspective and the clinical characterization of the cases: time of disease detection, forms of contamination, chronic noncommunicable diseases (CDNT's), co-infection, hepatitis B immunization, time of treatment, schedule of treatment, adverse effects, forgetfulness of dose, interruption of treatment, difficulty in obtaining medications; as well as an issue assigned to the expression of some difficulty in continuing the treatment.
Data collection was performed by nursing students who are part of the Evidence Based Nursing Studies and Research Group (GEPEBE), as fellows and volunteers, after a pre-test performed with three subjects. As no instrument changes were required, the pre-test interviews made up the final sample.
For the process of grouping the data, an electronic database was developed in the Microsoft Excel Program, 2010. The analysis, in turn, was performed through descriptive statistics, frequencies, mean and standard deviation in the SSPS, version 20.0 with the Chi-square test and Fisher's Exact test to evaluate the significance of the variables. The significance level adopted for the tests was p <0.05.
This study followed all the precepts of Resolution 466 of 2012 of the National Health Council, which deals with researches with human beings. In order to carry out the proposed steps, the institution was asked to consent to the research, and the project was submitted to the Ethics Committee of the Federal University of Rio Grande do Norte (UFRN), which approved it under opinion No. 056742/2015, CAAE 46207115.5.0000.5537.
The present study was developed in response to the project on Educational Actions on Chronic Hepatitis C: Empowering users, professionals and nursing students, who received funding from the Pró-Rectory of Extension of the Federal University of Rio Grande do Norte.
RESULTS
After analyzing the interviews, the subjects had a mean age of 57.11 years (SD ± 9.06), family income of 2.03 minimum wages (SD ± 1.71), time of infection detection of 6 , 37 years on average (SD ± 7.44) and mean treatment time of 1.98 years (SD ± 2.30). The other sociodemographic aspects are shown in Table 1.
Table 1. Sociodemographic characterization of patients with chronic hepatitis C. Natal/RN, 2017.
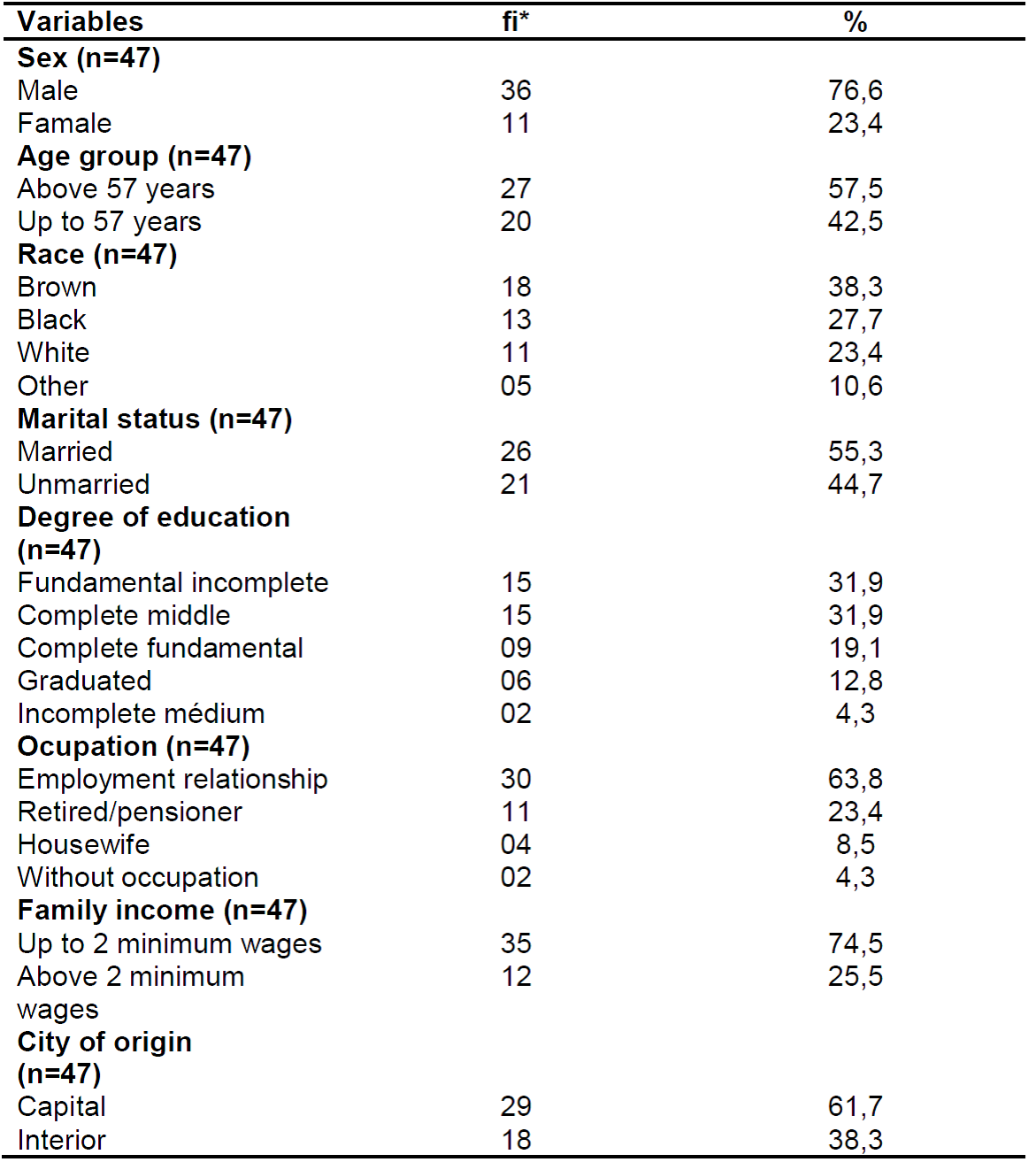
Source: own authorship, 2017.
*fi: absolute frequency.
As shown in Table 1, there is a predominance of males (76.6%), with a 57.3% age group, brown (38.3%), married civil status (55.3%), (31.9%), being active workers (63.8%), and the majority residing in Natal (61.7%).
According to the presentation of the clinical aspects of patients with chronic hepatitis C (Table 2), there is a predominance of up to 6 years of exposure (68.1%), lack of knowledge about contamination (57.5%), presence of CNCDs 53.4%), being the most frequent (64.3%), most of them did not present coinfection (91.5%) and were immunized against hepatitis B (65.9%). , 1%) with duration of up to 2 years (82.5%).
Table 2. Clinical characterization of patients with chronic hepatitis C. Natal/RN, 2017.
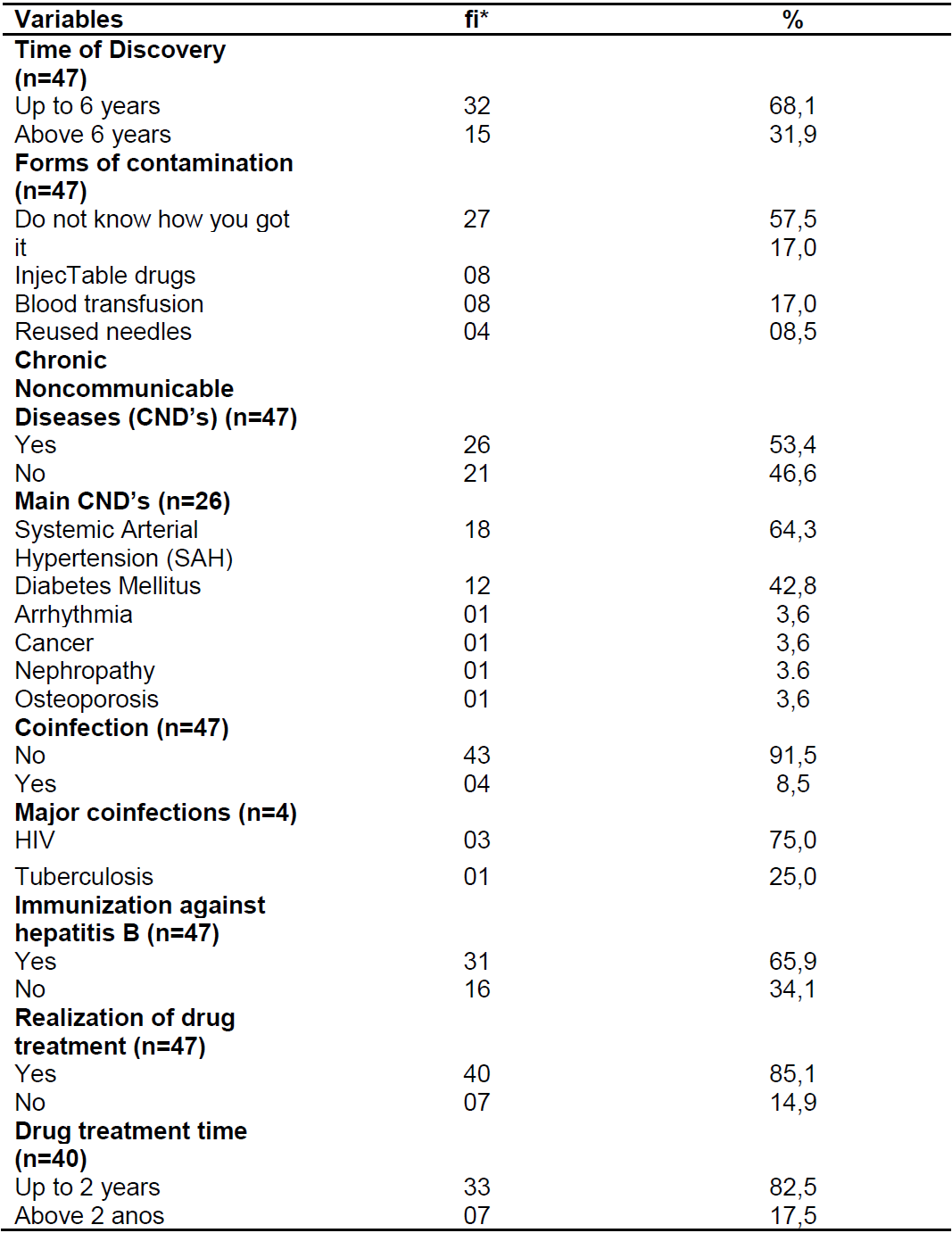
Source: Own authorship, 2017.
*fi: absolute frequency.
Among the sample of patients who undergo drug treatment, 57.4% of them are aware of the drug regimen used, in which the majority cited the drug Ribavirin (55.6%). Furthermore, 70.2% presented isolated or associated adverse effects, in which pain in the body was the most frequent adverse effect (30.3%). These aspects are detailed in Table 3.
Table 3. Distribution of the main drugs used and the adverse effects caused in patients with chronic hepatitis C. Natal, 2017.
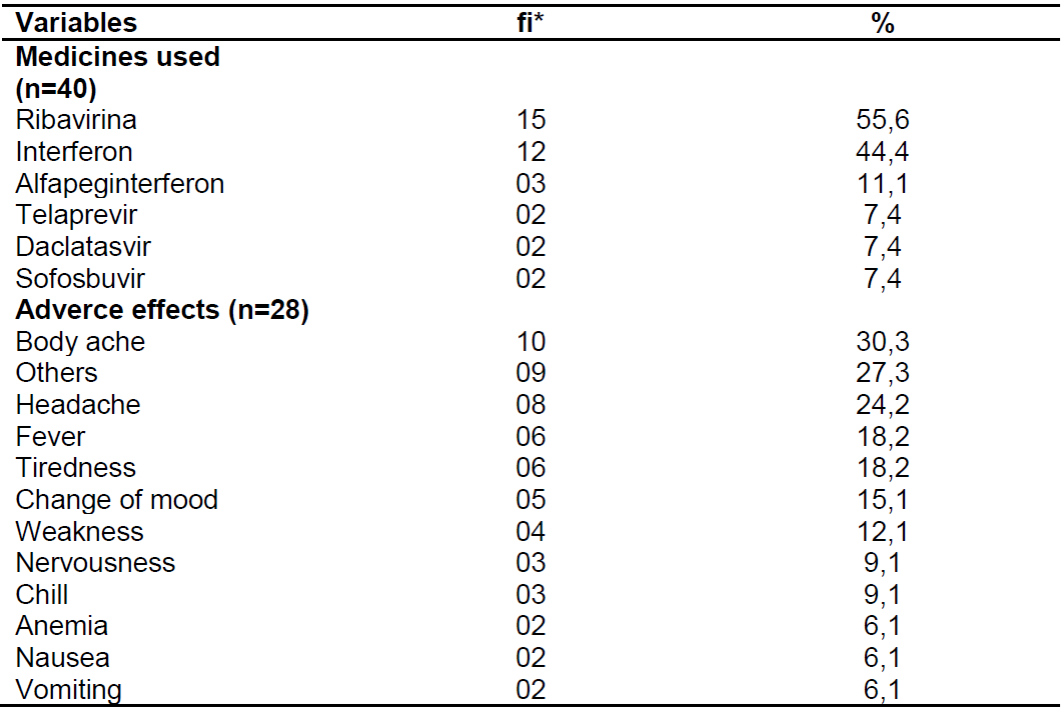
Source: Own authorship, 2017.
*fi: absolute frequency.
Regarding the aspects related to adherence to the drug, it was verified that 87.2% did not interrupt the treatment even though it had an adverse effect; 97.9% reported that when there are no symptoms, treatment does not end, that is, it concludes until there is a medical order; 84.6% reported difficulty in continuing treatment, as a consequence of personal aspects such as family relationship, their social value; 93.6% said they have no difficulty getting medicine.
Among the sociodemographic and clinical variables of the study, when they are statistically tested, it is possible to identify relevant associations that favor the characterization of the study, as shown in Table 4.
Table 4. Association between sociodemographic and clinical variables of patients with chronic hepatitis C. Natal, 2017.

Source: Own authorship, 2017.
(1)Qui-quadrado
(2)Fisher's exact test.
According to Table 4, when listing the city of origin with time of discovery, 83.3% of the participants from the countryside had up to 6 years of discovery. Regarding the relationship with the variables: age group and DCNT'S, it was observed that 74.1% of the population over 57 years old had CNCDs. Regarding the relationship between race and DCNT's, it was found that 84.6% of black participants had DCNT's.
Regarding the level of education and the variable knowledge of the treatment, it was observed that 100% of the patients with incomplete secondary education had knowledge about the treatment performed, while those with a fundamental incompleteness (33.3%) few had this knowledge. Finally, when relating the knowledge of the treatment and time of treatment, it was evidenced that 95.0% of those who did not have the knowledge had treatment time up to two years.
DISCUSSION
Regarding the gender variable, a predominance of males was verified, this is justified by the vulnerability of men to risk factors, such as injecting drug users, shown in a similar study in Bahia12.
Regarding the age group, those older than 57 years were predominant, which may be related to the advent of the increase in life expectancy that reaches the Brazilian population as a consequence of the technological advance of medicine as well as the expanded concept of health13.
In the racial aspect, the majority declared itself brown. Although there is a shortage of studies in this respect, the population base studied was the state of Rio Grande do Norte (RN) and, according to data from the Brazilian Institute of Geography and Statistics (IBGE), the state has a mostly brown population14.
As for marital status, it was observed that the sample had the majority of married subjects. This aspect shows a disagreement with another study, which showed a higher frequency of young single adults with multiple sexual partners, which caused the relation with the risk factors for the acquisition of the infection15.
Regarding the level of schooling, the patients presented with the incomplete elementary and middle level incomplete elementary level, agreeing only with incomplete primary education in another study12. However, the low level of schooling observed by this adult population leads to challenges for the multiprofessional team to clarify the disease and adhere to the treatment16.
Regarding the predominance of the discovery time in up to 6 years, it is related to the changes that the Ministry of Health performs in front of the diagnosis in which, recently, it was invested in new diagnostic methods of easy access, besides incentive to the education in health directed for this public6.
In immunization against hepatitis B, most are immunized, since treatment against C virus does not prevent hepatitis B. Thus, the hepatitis C carrier should be oriented on measures to prevent viral hepatitis and be immunized against hepatitis A and B7.
Regarding the form of contamination, it was predominantly unknown, since it may be related to the silent characteristic of the infection without the presence of signs and symptoms that characterize it, where the late manifestations result in the development of the chronic stage of the disease17,18.
Although cases of coinfection have not been predominant, it is known that cases of HCV coinfection are high, mainly HIV due to the form of parenteral contamination with a history of injecting drug use and blood transfusions19.
Among the individuals undergoing treatment, the standard regimen with Ribavirin and Interferon prevailed. Although they act to eradicate the virus, it is known that the new medications inserted into the clinical protocol and therapeutic guidelines promote a greater chance of cure in about 90 %, with lower adverse effects brings with it the consequences of adverse effects weakening the physical appearance of these users, through pain in the body, headache, fever and fatigue20,21.
It was observed an association between those over 57 years old and the presence of CNCDs, which is justified in a study with the Brazilian population, showing the SAH reaching about 40% of self-reported individuals22,23.
However, when associating the racial aspect with the presence of CNCDs, a higher prevalence in blacks was observed, since this population group presents greater genetic predispositions for the presence of SAH, mainly23.
To the level of schooling, this is directly related to the predominant family income of up to 2 minimum wages, since the employment bonds are more judicious for the occupation of jobs with better remunerations24.
When associated to the variables, city of origin and time of discovery, it was observed that the carriers coming from the interior have less discovery time than those coming from the capital, which disagrees with the evidence found in other studies, which address government planning as a variable which interferes with accessibility to health services25.
CONCLUSION
The present study characterized the profile of patients with chronic hepatitis C in an outpatient clinic in Natal / RN, composed of men, with adult age, married, with low level of schooling, active workers, born in the capital. Regarding the clinical situation, they were infected for less than 6 years of discovery, not knowing the form of contamination, in drug treatment with Ribavirin and Interferon in about 2 years of treatment, presenting side effects, but not ceasing treatment in face of these effects.
Among the limitations of the study are the lack of control of the patients who attend the service, since the only form of control is the appointment schedule available at reception, which makes it difficult to recruit participants during data collection.
Sociodemographic and clinical characterization assist the clinical practice of the multiprofessional team with patients with chronic hepatitis C. Therefore, it is suggested a standardization of the control of patients followed at the outpatient clinic, besides the urge to carry out new studies that better cover the epidemiology of patients with hepatitis C.
REFERENCIAS
1. Joukar F, Mansour-Ghanaei F, Naghipour MR e Hasandokht T. Nurses' Knowledge toward Hepatitis B and Hepatitis C in Guilan, Iran. The Open Nursing Journal [on line]. 2017; [citado 08 jun 17] 11(8). Disponível em: https://benthamopen.com/FULLTEXT/TONURSJ-11-34 [ Links ]
2. WHO (World Health Organization). Manual for the development and assessment of national viral hepatitis plans. Geneva, 2015; [citado em 08 jun 17] [ Links ]
3. Vijgen L, Thys K, Vandebosch A, Van Remoortere P, Verloes R, De Meyer S. Virology analysis in HCV genotype 1-infected patients treated with the combination of simeprevir and TMC647055/ritonavir, with and without ribavirin, and JNJ-56914845. Virology jornal [online]. 2017; [citado 08 jun 17] 14. Disponível em: https://virologyj.biomedcentral.com/articles/10.1186/s12985-017-0760-2 [ Links ]
4. Ministério da Saúde (BR). Boletim Epidemiológico Hepatites Virais. 5ª ed. Brasília (DF): Ministério da Saúde; 2016. [ Links ]
5. Fagundes GD, Bonazza V, Ceretta LB, Back AJ, Betiol J. Detecção do vírus da hepatite c em uma população de adultos. Rev Latino-Am Enfermagem [on line]. 2008 mai/jun; [citado 2017 fev 09]; 16(3). Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-11692008000300010&lng=pt&nrm=iso&tlng=pt [ Links ]
6. Fonseca, JCF. Histórico das hepatites virais. Rev Soc Bras Med Trop Uberaba [on line]. 2010 jun; [citado 2017 fev 09] 43(3):[aprox. 8 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822010000300022&lng=pt&nrm=iso [ Links ]
7. Ministério da Saúde (BR). Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções. 1ª ed. Brasília (DF): Ministério da Saúde; 2015. [ Links ]
8. Velosa J, Serejo F, Ramalho F, Marinho R, Rodrigues B, Baldaia C, Raimundo M, Ferreira P. A practical guide for antiviral therapy of chronic Hepatitis C. Sociedade Portuguesa de Gastrenterologia/ SCOPUS [periódico na internet]. 2014 nov; [citado 2017 mai 14]; 21(6):[aprox.9 telas]. Disponível em: http://ac.els-cdn.com/S234145451400115X/1-s2.0-S234145451400115X-main.pdf?_tid=357e6e7c-3908-11e7-93cd-00000aab0f6b&acdnat=1494809487_49483b3fee6a80d24af010126eda99e0 [ Links ]
9. Amorin, S., Oliveira, R. Controle da sintomatologia para o aumento da adesão à terapêutica no tratamento da hepatite C. Fonseca Online. Rev Clin Hosp Prof Dr Fernando Fonseca [periódico na internet]. 2013; [citado 2017 fev 09] 1(1): 19-22. 2013. Disponível em: http://hdl.handle.net/10400.10/977 [ Links ]
10. Ministério da Saúde (BR). Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções. 1ª ed. Brasília (DF): Ministério da Saúde; 2011. [ Links ]
11. Hulley SB. Delineando a pesquisa clínica: uma abordagem epidemiológica. Artmed. 2015. [citado em 08 jun 17] 4. [ Links ]
12. Moraes MTM, Oliveira TJ. Perfil epidemiológico e sóciodemográfico de portadores de hepatite c de um município do sudoeste baiano [trabalho de conclusão do curso]. Santa Cruz do Sul (RS): Repositório, Universidade de Santa Cruz do Sul; 2016. [ Links ]
13. Camargos MCS, Gonzaga MR. Viver mais e melhor? Estimativas de expectativa de vida saudável para a população brasileira. Cad Saúde Pública [onlinet]. 07/15 [citado 08 jun 17]; 31(7): 12. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2015000701460&lng=en [ Links ]
14. BRASIL. Ministério do Planejamento, Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística. Censo demográfico. 2010 [online] Disponível em: http://www.ibge.gov.br/estadosat/temas.php?sigla=rn&tema=censodemog2010_snig [ Links ]
15. Neto JR, Cubas MR, Kusma SZ, Olandoski M. Prevalência da hepatite viral C em adultos usuários de serviço público de saúde do município de São José dos Pinhais - Paraná. Rev bras epidemiol. [on line]. 2012 Set [citado 2017 Mai 18]; 15(3):[aprox 9 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-790X2012000300016&lng=en [ Links ]
16. Rodrigues FFL, Santos MA, Teixeira CRS, Gonela JT, Zanetti ML. Relação entre conhecimento, atitude, escolaridade e tempo de doença em indivíduos com diabetes mellitus. Acta paul enferm. [online]. 2012 [citado 08 jun 17]; 25(2):[aprox 6 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-21002012000200020&lng=en http://dx.doi.org/10.1590/S0103-21002012000200020 [ Links ]
17. Kubota K, Campos MAS, Pereira LRL. Análise da Assistência a Saúde aos Pacientes com Hepatites Virais B e C no Estado do Amapá. Revista de Ciências Farmacêuticas Básica e Aplicada [on line]. 2014 set; [citado 2017 mai 14]; 34(4):[aprox 8 telas]. Disponível em: http://serv-bib.fcfar.unesp.br/seer/index.php/Cien_Farm/article/viewFile/3199/3199 [ Links ]
18. Umumararungu E, Ntaganda F, Kagira J, Maina N. Prevalence of Hepatitis C Virus Infection and Its Risk Factors among Patients Attending Rwanda Military Hospital, Rwanda. BioMed Research International [on line]; 2017 [citado em 2017 mai 14]; [aprox.7 telas] Disponível em: https://doi.org/10.1155/2017/5841272 [ Links ]
19. Martins T, Narciso-Schiavon JL, Schiavon LL. Epidemiologia da infecção pelo vírus da hepatite C. Revista da Associação Médica Brasileira [on line]; 2011 Jan/Fev; [citado 2017 abril 9]; 57(1):[aprox 5 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302011000100024 [ Links ]
20. Rêgo et al. Estudo comparativo entre ensaios sorológicos utilizados no diagnóstico de hepatite c no laboratório central de saúde pública de macapá - amapá. Ciência Equatorial. [online] 2013, [citado 08 jun 17]; 3(1): [aprox 6 telas] Disponível em: https://periodicos.unifap.br/index.php/cienciaequatorial/article/view/803/MarlissonO [ Links ]
21. Kunrath AAF, Junges JR, López LC. Vulnerabilidades e subjetividades de pessoas com diagnóstico e tratamento de hepatite C. Saúde debate [on line]; 2014 Abril/Jun [citado 2017 mai 14]. 38(101):[aprox 8 telas]. Disponível em: http://www.scielo.br/pdf/sdeb/v38n101/0103-1104-sdeb-38-101-0225.pdf [ Links ]
22. Andrade SSA, Stopa SR, Brito AS, Chueri PS, Szwarcwald CL, Malta DC. Prevalência de hipertensão arterial autorreferida na população brasileira: análise da Pesquisa Nacional de Saúde, 2013. Epidemiol Serv Saúde [on line]. 2015 Jun [citado 2017 Maio 18]; 24(2):[aprox 7 telas]. Disponível em: http://scielo.iec.pa.gov.br/scielo.php?script=sci_arttext&pid=S1679-49742015000200012&lng=pt [ Links ]
23. Martins T, Narciso-Schiavon JL, Schiavon LL. Epidemiologia da infecção pelo vírus da hepatite C. Revista da Associação Médica Brasileira [on line]; 2011 Jan/Fev; [citado 2017 abril 9]; 57(1):[aprox 5 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302011000100024 [ Links ]
24. Balassiano M, Seabra AA, Lemos AH. Escolaridade, salários e empregabilidade: tem razão a teoria do capital humano?. Rev adm contemp [online]. 2005 [citado 08 jun 17]; 9(4): [aprox 21 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-65552005000400003&lng=en&nrm=iso [ Links ]
25. Lima LD, Viana AL, Machado CV, Albuquerque MV, Oliveira RG, Iozzi FL et al . Regionalização e acesso à saúde nos estados brasileiros: condicionantes históricos e político-institucionais. Ciênc saúde coletiva [online]. 2012 Nov [citado 09 Jun 2017 ]; 17(11):[aprox.11 telas]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-81232012001100005&lng=pt [ Links ]
Received: January 04, 2018; Accepted: March 02, 2018











 texto en
texto en 


