Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Enfermería Global
versión On-line ISSN 1695-6141
Enferm. glob. vol.20 no.64 Murcia oct. 2021 Epub 25-Oct-2021
https://dx.doi.org/10.6018/eglobal.450831
Originals
Nursing diagnoses related to the potential adverse effects of antineoplastic chemotherapyia
1 Departamento de Enfermería Básica. Escuela de Enfermería, Universidad Federal de Minas Gerais, Belo Horizonte, MG, Brasil. lorena.medreiros@gmail.com
2 Departamento de Enfermería, Facultad de Ciencias de la Salud, Universidad de Brasília, Brasília, DF, Brasil.
Objective:
To identify possible nursing diagnoses related to the adverse effects of antineoplastic chemotherapy in cancer patients.
Method:
Quantitative, descriptive study, performed at the chemotherapy ambulatory of a public hospital, in Belo Horizonte, Minas Gerais, Brazil. Seventy patients were included in the sample and interviewed to obtain sociodemographic characteristics. Clinical data and the chemotherapy protocol were obtained from the physical record. Possible toxicities and adverse effects were identified for each chemotherapy protocol through a textbook and, subsequently, nursing diagnoses were identified in the taxonomy of the North American Nursing Diagnosis Association International (NANDA-I), version 2018-2020, and associates toxicities and adverse effects.
Results:
The most prevalent primary site was colon and rectum (30%), and breast (30%). All participants were receiving chemotherapy with potential hematological, gastrointestinal, cardiovascular, and dermatological toxicity. Thirty-six nursing diagnoses were identified based on NANDA-I, with a greater predominance of diagnoses in the safety/protection domain, and the elimination and exchange domain.
Conclusion:
The identification of Nursing Diagnoses based on chemotherapy protocols allows the proposition of individualized care plans to meet the needs of patients undergoing chemotherapy, with a focus on preventing the occurrence and minimizing adverse effects, when already present.
Keywords: Nursing Diagnosis; Oncology Nursing; Adverse Effects; Chemotherapy; Nursing Process
INTRODUCTION
Chemotherapy (CHT) is one of the modalities of systemic cancer treatment performed through the administration of cytotoxic drugs, either alone or in combination, which targets cancer cells1,2. However, CHT has nonspecific action on cells with a high proliferative capacity, that is, it acts on both cancer cells and normal cells that have rapid renewal. Thus, it causes toxicities to normal tissues that are manifested by adverse effects due to CHT1,2.
Acute adverse effects (AE) can appear during the drug administration period and up to 24 hours after the end or they can appear later, taking months or years to manifest3. The most common AEs are nausea, vomiting, inappetence, diarrhea, constipation, weakness, fatigue, alopecia, anemia, neutropenia, among others2,4. These AEs can generate physical, psycho-emotional, social, spiritual, and economic damages5.
Thus, cancer patients undergoing CHT require care aimed at preventing and controlling AEs to ensure a better quality of life and continuity of treatment1)(2)(6. Nurses have an important role in the prevention, identification, management, and control of AEs related to CHT7. To guide the management of nursing care, they use the Nursing Care Systematization (NCS) as a tool.
According to Resolution 358/2009 of the Federal Council of Nursing (COFEN) of Brazil, NCS is a working methodology of Nurses, carried out through the Nursing Process, which occurs in five stages: Nursing history (anamnesis and physical examination); Nursing Diagnoses (ND); nursing care planning (expected results and actions to be taken); implementation of care and assessment of the effectiveness of actions8. Within the Nursing Process, diagnostic reasoning allows the construction of the nursing care and interventions plan. In the clinical practice of Nursing in Oncology, NDs will guide prophylactic and/or therapeutic actions, health education/guidance for the prevention and management of signs and symptoms of the disease, AE related to CHT, and actions regarding the individual's psychosocial and family needs5.
There are several studies aimed at identifying possible ND-related AEs of treatments in cancer patients7)(9)(10. Carvalho et al9 carried out a study seeking to build NDs applicable to oncohematological patients, who have post-CHT AEs, based on terms identified by oncology nurses in the International Classification for Nursing Practice (ICNP®). Sousa et al10 evaluated medical records of oncohematological patients to identify possible terms in the nursing records that were comparable to NDs, according to NANDA Taxonomy II. Another study sought to identify the most frequent ND in oncohematological patients, submitted only to CHT, based on the analysis of medical records7. The knowledge of the priority NDs for a specific patient helps the nurses' diagnostic reasoning11.
Although these studies have data regarding ND related to AEs in CHT, the results are based on retrospective AEs data that patients with a certain type of cancer presented at the end of treatment. No study has carried out a survey of possible NDs related to potential acute AEs in cancer patients who are receiving CHT associated or not with other oncological therapies.
Thus, this study aims to identify possible Nursing Diagnoses related to the acute adverse effects of chemotherapy in cancer patients.
MATERIAL AND METHOD
Study design
This is an observational, descriptive cross-sectional study with a quantitative approach, carried out at the chemotherapy outpatient clinic of a public university and large hospital, with care exclusively through the Unified Health System (SUS), located in Belo Horizonte, Minas Gerais, Brazil.
Population and sample
We selected patients by convenience sample, according to the following eligibility criteria: people aged 18 years old or older, diagnosed with malignancy, and undergoing outpatient CHT during the data collection period. We excluded patients with physical and mental incapacity to communicate, as they presented drowsiness due to the effects of medications hindering to conduct of interviews, and that physical records were unavailable for consultation. The sample consisted of 70 patients.
Data collect
Data collection was performed from October 2017 to May 2018. The study was performed in three stages. Stage 1: An interview with the patients included in the study to obtain the sociodemographic data of the sample; Stage 2: Access to the patients' medical records to obtain data regarding the clinical condition and the CHT protocol; and Stage 3: a review of the literature on possible toxicities and AEs related to the CHT protocol, and possible NDs. The authors built a semi-structured and adapted instrument, used as a guide for data collection, containing: sociodemographic data (gender, self-reported skin color, age, and civil status), clinical data (primary tumor site, disease stage, presence of metastasis, site of metastasis, and other concomitant treatments, such as surgery and/or radiotherapy), data related to CHT (protocol - monotherapy/polytherapy, CHT in use, pre-CHT medications).
We identified the possible toxicities and AEs of each patient based on the textbook, reference in oncology in Brazil, entitled “Terapêutica oncológica para enfermeiros e farmacêuticos 4) based on the drugs that were part of the CHT protocol of each patient in the sample. Sociodemographic and clinical data were not associated with possible toxicities and were intended only to characterize the sample. Toxicities were categorized into: neurological, pulmonary, cardiovascular, gastrointestinal, hepatic, vesical/renal, reproductive, metabolic, hematological, dermatological, anaphylactic reactions, and fatigue. Subsequently, we identified the possible AEs for each toxicity related to CHT.
Finally, we elaborated the possible NDs according to the taxonomy of the North American Nursing Diagnosis Association International (NANDA-I) version 2018-202011. The possible NDs were structured according to the toxicity and AEs that could cause them and presented within each domain to which it belongs in the NANDA-I taxonomy.
Statistical analysis
We performed a quantitative and descriptive analysis of the study variables using the Statistical Package for Social Science (SPSS) software, version 19.0. Absolute (AF) and relative (RF) frequencies were calculated for sociodemographic, clinical, and possible identified toxicities, as well as measures of central tendency (mean and median) and dispersion (standard deviation, minimum and maximum) when applicable. Possible NDs have been described qualitatively and associated with potential AES.
Ethical considerations
The study is part of the project entitled “Integrative and complementary health practices: evidence for care in oncology”, which was approved with CAAE number 66568117.1.0000.5149 by the Research Ethics Committee - COEP of the Federal University of Minas Gerais and by the Hospital das Clínicas/EBSERH, according to the resolution of the National Health Council 466/201212. All participants signed an informed consent form (ICF).
RESULTS
The sample was composed of 58.6% of female patients, the majority declared themselves brown and 52.9% were married. The average age was 53.8 years and the highest proportion of patients was in the 40-59 age group. Table 1 shows the sociodemographic data of the study participants.
Table 1. Sociodemographic characteristics of the sample (n = 70). Belo Horizonte - MG, Brazil, 2019.
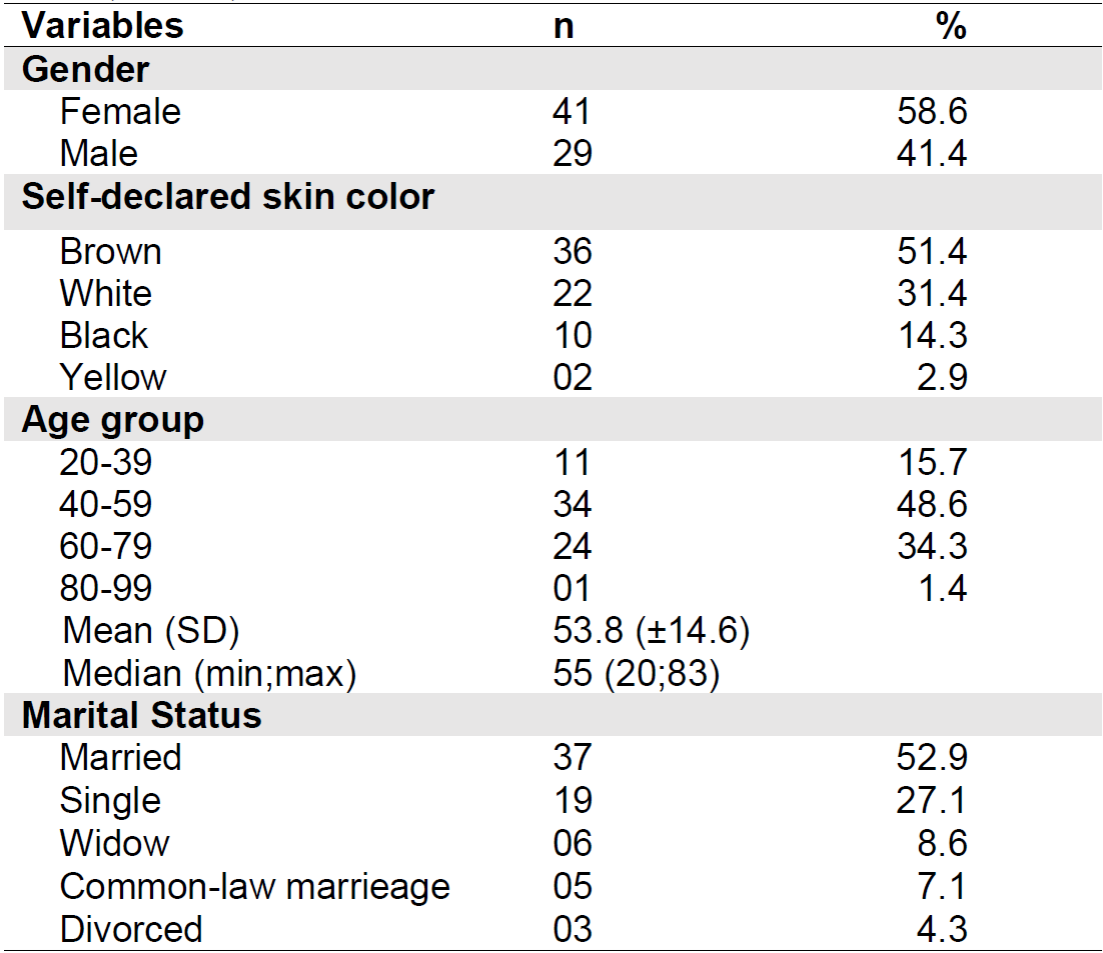
Note: SD= Standard Deviation
Regarding the clinical characteristics of the patients, the most prevalent primary cancer site was colon and rectum, and breast, both with a relative frequency of 30%. Stage IV was present in most (51.4%) of the sample. Metastasis was present in 55.7% of patients, 28.2% in more than one site and 33.3% had unknown sites. The lung was the organ most affected (7.6%) by metastasis.
In addition to CHT, 80% (n = 56) of the sample underwent surgery and 34.3% (n = 24) underwent radiotherapy. We should highlight that 14.3% (n = 10) of the sample underwent the three treatment modalities for cancer. Table 2 shows the clinical data related to the diagnosis of cancer and treatment of the sample.
Table 2. Clinical characteristics of the sample (n = 70). Belo Horizonte - MG, Brazil, 2019.
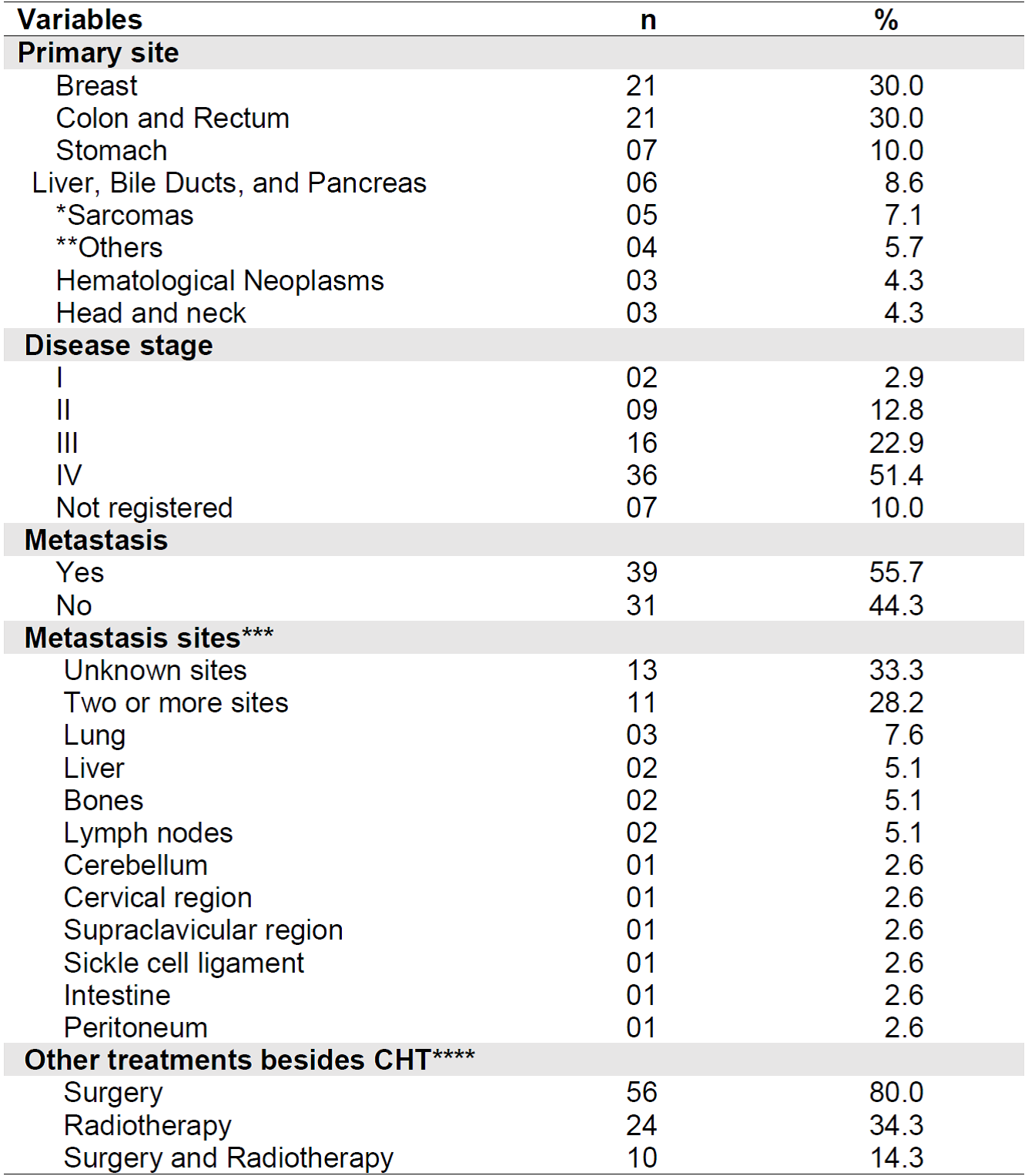
Notes: SD= Standard Deviation; CHT = Antineoplastic chemotherapy;
*Sarcomas= Kaposi's sarcoma, synovial sarcoma, soft tissue sarcoma, osteosarcoma; **Others= prostate, ovary, lung (2), cervical neuroblastoma.
*** Percentage calculated based on the total number of patients who had metastasis.
**** Percentage calculated considering the total sample n = 70;The patient may have undergone more than one therapy option.
All individuals who received CHT for cancer treatment were submitted to pre-medication (pre-CHT) protocols. In this study, the drugs most used in pre-CHT were ondansetron (94.3%), dexamethasone (81.4%), ranitidine (60.0%), folic acid (32.8%), diphenhydramine (22.8%), mannitol (15.7%), and metoclopramide (14.3%). It should be noted that the same patient used one or more pre-CHT medications.
Polychemotherapy was predominant (70%), with 32.7% undergoing the protocol known as Folfox (oxaliplatin, 5-fluorouracil (5-FU), and leucovorin). Table 3 shows information related to antineoplastic treatment protocols.
Table 3. Distribution of study participants according to antineoplastic chemotherapy protocol and potential toxicities (n = 70). Belo Horizonte - MG, 2019.
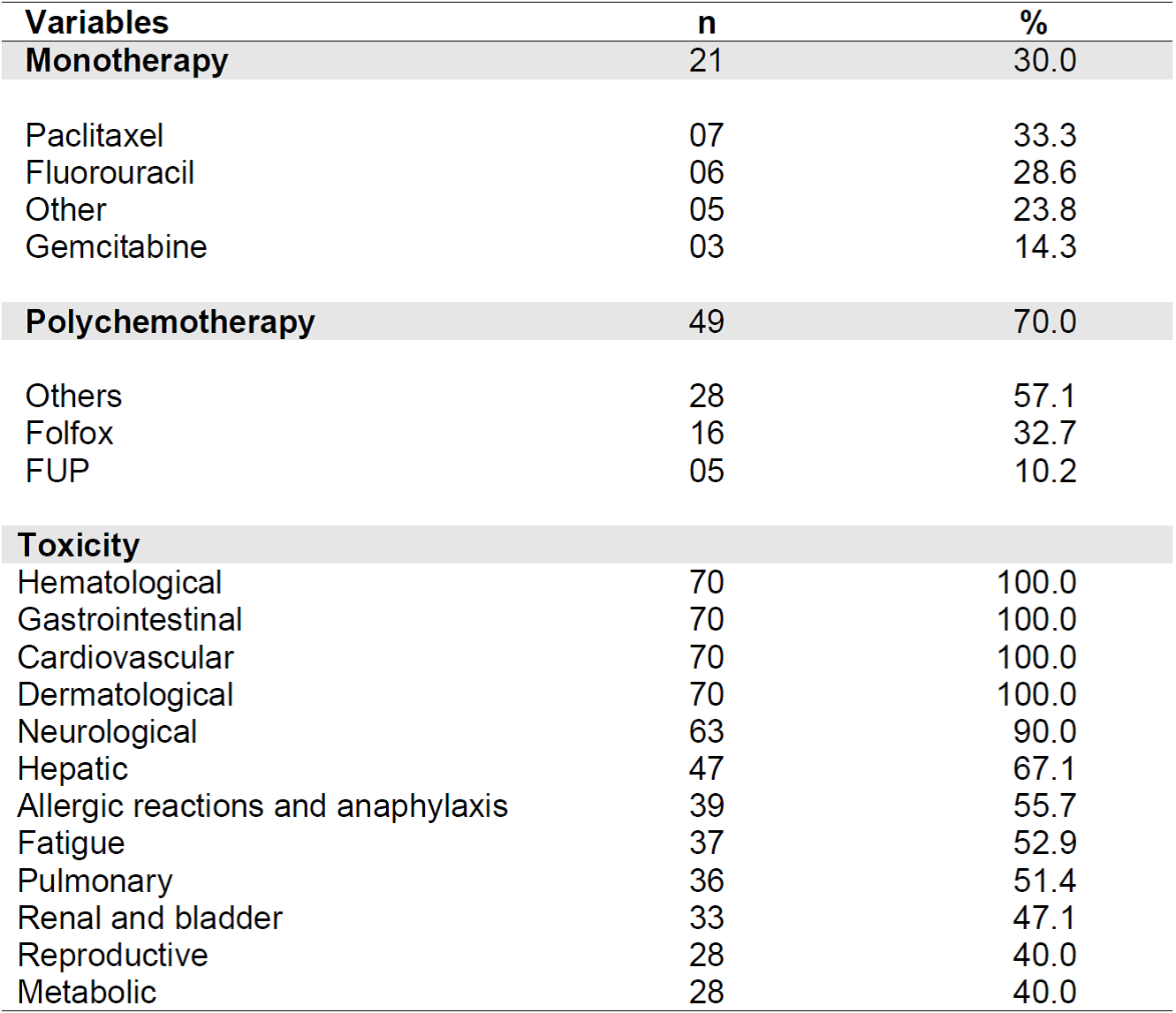
All participants have potential hematological toxicity (leukopenia, thrombocytopenia and/or anemia), cardiovascular (acute electrocardiographic changes such as arrhythmias, and chronic changes such as congestive heart failure); gastrointestinal (nausea, vomiting, mucositis, diarrhea, and constipation); local dermatological (phlebitis, pain, erythema and tissue necrosis due to drug extravasation) and systemic (alopecia, nail changes, urticaria, skin rash, hand-foot syndrome, hyperpigmentation, and photosensitivity) throughout the treatment, among other signs and symptoms.
Neurological damage was a frequent AE (90%), which can be mild or severe, transient or permanent, such as encephalopathy (confusion, agitation, dizziness), peripheral neuropathy (paraesthesia, muscle weakness, impotence, decreased reflexes), cranial neuropathy (ototoxicity, taste change), autonomic neuropathy (constipation, urinary changes, bladder atony), cerebellar syndromes (dysmetria, ataxia, nystagmus, vertigo), among others.
Table 4 shows the 36 possible NDs identified, categorized according to general toxicities and their possible AE.
Table 4. Nursing diagnoses according to the adverse events induced by antineoplastic chemotherapy according to the NANDA-I taxonomy (2018-2020)11. Belo Horizonte, MG, 2019.


Note: NANDA-I = North American Nursing Diagnosis Association International;
* Nursing diagnoses that are repeated in other toxicity.
AEs have systemic repercussions, so the same AE was associated with more than one domain of the NANDA-I Taxonomy11 and, consequently, generated more than one ND. Some domains and NDs have been correlated to more than one EA, which is why they appear more than once. The domain of safety/protection is the one that has more NDs (n = 12) and is related to six toxicities, and the domain of elimination and exchange is the second most present in a quantity of ND identified, with eight NDs related to four toxicities.
DISCUSSION
This study aim to identify possible NDs based on the characteristics of the CHT protocols of the patients included. There was a predominance of females (58.6%) in the sample. Similarly, data in the literature show the presence of 50% or more of the diagnosis of cancer being composed of women13. The most prevalent primary sites in the sample were the breast, colon, and rectum. Estimates for the 2020-2022 three-year period point to female breast cancer as the most frequent, except non-melanoma skin cancer, in all Brazilian regions, and colon and rectal cancer has an approximate risk estimate for men and women14, corroborating the predominance of women in the sample.
Regarding the age group, the largest proportion of patients was between 40-59 years old (48.6%), with an mean of 53.8 years old. Oliveira et al15 found that the average age of the first cancer diagnosis in Brazil was 51.9 years old. Data from the National Cancer Institute José Alencar Gomes da Silva (INCA) also indicate that most people with cancer are in the middle age group14. Even with screening and early cancer detection technologies, stage IV was the most prevalent (51.4%) and most (55.7%) of the sample has metastasis. Cancer staging predicts the extent and severity of cancer and is a basis for defining treatment1. According to the Ministry of Health, 60% of cancer patients in Brazil are diagnosed at an advanced stage of the disease (III and IV)16.
In addition to CHT, 80% of people underwent surgery, 34.3% underwent radiotherapy and 14.3% underwent the three treatment modalities. Regarding the protocols, there was a predominance of polychemotherapy (70%), and the most prevalent was the Folfox protocol (n = 16; 32.7%). This protocol is widely used to treat colon and rectal cancer4)(17)(18, which was one of the most frequent in the sample.
Regarding the possible toxicities according to the sample's CHT protocols, all participants have potential hematological, gastrointestinal, cardiovascular, and dermatological toxicity, in addition to 90% presenting potential neurological damage. Studies demonstrate that the management of AEs is still not adequate in healthcare practice, as the symptoms are not well managed or are underestimated to signs and symptoms already expected by the treatment19,20. AEs are elements that limit the continuity of treatment and have a great impact on patients' quality of life2,6.
Nurse assistance in CHT encompasses a series of activities, following COFEN Resolution 569/2018, which allows the applicability of NCS, acting in the planning, organization, execution, supervision, and evaluation of the care of patients undergoing cancer treatment and performance in the prevention, management, and treatment of AEs21.
The accuracy of the identification of the possible AEs of cancer treatments is a valuable tool for designing nursing care aimed at preventing, minimizing, and resolving AEs, even though they are considered ineviTable due to the cytotoxicity of the drug 20. This is because the absence of adequate measures for the management of AEs by the service assistance team becomes a limiting element in the continuity of treatment, precisely because it impacts the quality of life of people with cancer2,6.
The implementation of NP for cancer patients undergoing CHT allows the construction of nursing care plans based on scientific evidence and is reliable to the patient's needs, focusing on assisting in a humanized, holistic, qualified, and competent way22. In this process, ND is fundamental, as they are listed based on the nurse's clinical reasoning and from them, care will be guided in an appropriate and individualized way11,23.
In this study, we were able to identify 36 NDs based on the AEs of the CHT. The domains with the most predominance of NDs were safety/protection, and elimination and exchange. A study performed of a university hospital in Rio de Janeiro to identify the care needs of cancer patients and to correlate these needs with the domains of the NANDA taxonomy, managed to identify the domains: nutrition; elimination and exchange; activity/rest; role relationship; coping/stress tolerance23. These results corroborate what we found in this study.
Sousa et al10 identified 30 NDs by mapping the medical records of onco-hematological patients at a clinic in Rio de Janeiro. Comparing with the NDs found in this study, we have in common the identification of the diagnoses of Risk for infection, Risk for bleeding, Risk for impaired skin integrity, Risk for falls, Constipation, Risk for constipation, Urinary retention, Acute pain, Fatigue, Nausea, and Imbalanced nutrition, less than body needs10. Most studies that seek to identify NDs applicable in cancer patients target the sample for patients with specific types of cancer7)(9)(10. Recent research on NDs is scarce in the literature.
Thus, the care for cancer patients is complex, as the needs may arise from psychosocial, spiritual issues, illness, CHT, and AEs caused. Therefore, the identification of NDs allows to set goals in the care process and, for this, professionals must know and be trained as to the methodology and theoretical framework that NCS and NP require in its implementation and execution22,23.
The lack of identification of the defining characteristics of patients in CHT is a limitation of this study. However, we aim only to identify NDs. The absence of current literature on the application of NDs in oncology was also a limiting factor in this study.
CONCLUSIONS
The identification of potential NDs based on the CHT protocols allows to propose of care plans to meet the needs of cancer patients, mainly with a focus on preventing the occurrence and minimizing AEs. In this study, we identified 36 NDs according to the NANDA-I Taxonomy based on the possible EA of CHT for our sample, which had predominantly patients with breast and colon, and rectal cancer.
Therefore, due to the various impacts and vulnerabilities that the AES can cause in the individual's life, it is important that Nursing professionals recognize the potential AES and apply the NCS using the NP as their theoretical-methodological reference, with identification of NDs and care planning, to act in the prevention, monitoring, and management of the AEs of CHT. An appropriate management of AE improves the quality of life and continuity of treatment. Finally, we expect that this work will contribute to the improvement of nursing practice in Oncology and promote reflection on the applicability of NCS and NP in the care of cancer patients receiving CHT.
REFERENCIAS
1. Leite MAC, Nogueira DA, Terra FS. Aspectos sociais e clínicos de pacientes oncológicos de um serviço quimioterápico. Revista Rene. 2015; 16(1):38-45. Disponível em: http://www.periodicos.ufc.br/index.php/rene/article/download/2661/2046 [ Links ]
2. Guimarães RCR, Gonçalves RPF, Lima CA, Torres MR, Silva CSO. Nursing actions facing reactions to chemotherapy in oncological patients. Revista Online de Pesquisa: Cuidado é Fundamental Online. 2015; 7(2):2440-52. Disponível em: http://www.seer.unirio.br/index.php/cuidadofundamental/article/view/3589/pdf_1559 [ Links ]
3. Jesus LG, Cicchelli M, Martins GB, Pereira MCC, Lima HS, Medrado ARAP. Repercussões orais de drogas antineoplásicas: Uma revisão de literatura. RFO UPF. 2016; 21(1):130-5. Disponível em: http://revodonto.bvsalud.org/pdf/rfo/v21n1/a20v21n1.pdf [ Links ]
4. Bonassa EMA, Gato MIR. Terapêutica Oncológica para enfermeiros e farmacêuticos. 4ª ed. São Paulo (SP): Editora Atheneu; 2012. [ Links ]
5. Matoso LML, Rosário SSD, Matoso MBL. As estratégias de cuidados para o alívio dos efeitos adversos da quimioterapia em mulheres. Saúde (Santa Maria). 2015; 41(2):251-60. Disponível em: https://periodicos.ufsm.br/revistasaude/article/download/10883/pdf#:~:text=Nesse%20sentido%2C%20%C3%A9%20de%20suma,contato%20com%20pessoas%20doentes%2C%20manter [ Links ]
6. Cunha FF, Vasconcelos EV, Silva SED, Freitas KO. Representações de pacientes oncológicos sobre o tratamento de quimioterapia antineoplásica. Rev Fund Care Online. 2017; 9(3):840-7. Disponível em: http://www.seer.unirio.br/index.php/cuidadofundamental/article/view/5579/pdf_1 [ Links ]
7. Calegari IB, Cordeiro ALPC, Stacciarini TSG, Ferreira LA. Diagnósticos de enfermagem em pacientes onco hematológicos submetidos a tratamento quimioterápico. Revista de Enfermagem e Atenção à Saúde [online]. 2018; 7(3):102-15. Disponível em: http://seer.uftm.edu.br/revistaeletronica/index.php/enfer/article/view/3116/pdf [ Links ]
8. Brasil. Resolução COFEN n. 358/2009, de 15 de outubro de 2009. Dispõe sobre a Sistematização da Assistência de Enfermagem e a implementação do Processo de Enfermagem em ambientes públicos ou privados, em que ocorre o cuidado profissional de Enfermagem. Conselho Federal de Enfermagem. Disponível em: http://www.cofen.gov.br/resoluocofen-3582009_4384.htm [ Links ]
9. Carvalho MWA, Araújo AA, Nóbrega MML. Diagnósticos de enfermagem para pacientes com toxicidade hematológica pós-quimioterapia antineoplásica com base na CIPE(r). Rev enferm UFPE on line. 2009; 3(4):801-7. Disponível em: https://periodicos.ufpe.br/revistas/revistaenfermagem/article/view/5570/4790 [ Links ]
10. Sousa RM, Santo FHE, Santana RF, Lopes MVO. Diagnósticos de enfermagem identificados em pacientes onco-hematológicos: mapeamento cruzado. Esc. Anna Nery. 2015; 19(1):54-65. Disponível em: https://www.scielo.br/pdf/ean/v19n1/1414-8145-ean-19-01-0054.pdf [ Links ]
11. Herdman TH, Kamitsuru S. Diagnósticos de enfermagem da NANDA: definições e classificação 2018-2020. 11ª ed. Porto Alegre: Artmed; 2018. [ Links ]
12. Brasil. Resolução n. 466/2012, de 12 de dezembro de 2012. Dispõe sobre as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Conselho Nacional de Saúde. Disponível em: https://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.html [ Links ]
13. Gonçalves MM, Guedes NAB, Matos SS, Tiensoli SD, Simino GPR, Corrêa AR. Perfil dos Atendimentos a Pacientes Oncológicos em uma Unidade de Pronto Atendimento. Revista de Enfermagem do Centro-Oeste Mineiro. 2018; 8:e2595. Disponível em: http://seer.ufsj.edu.br/index.php/recom/article/view/2595/1938 [ Links ]
14. Brasil. Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. Estimativa 2020: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA, 2019. [acesso em 17 jul 20]. 122 p. Disponível em: https://www.inca.gov.br/publicacoes/livros/estimativa-2020-incidencia-de-cancer-no-brasil [ Links ]
15. Oliveira MM, Malta DC, Guauche H, Moura L, Silva GA. Estimativa de pessoas com diagnóstico de câncer no Brasil: dados da Pesquisa Nacional de Saúde, 2013. Revista Brasileira de Epidemiologia. 2015; 18(2):146-57. Disponível em: https://www.scielo.br/pdf/rbepid/v18s2/1980-5497-rbepid-18-s2-00146.pdf [ Links ]
16. Brasil. Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. Estimativa 2018: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA, 2017. [acesso em 31 mar 19]. 130 p. Disponível em: https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2019/10/estimativa-incidencia-de-cancer-no-brasil-2018.pdf [ Links ]
17. Silva AA, Carlotto J, Rotta I. Padronização da ordem de infusão de medicamentos antineoplásicos utilizados no tratamento dos cânceres de mama e colorretal. Einstein (São Paulo). 2018; 16(2):1-9. Disponível em: https://www.scielo.br/pdf/eins/v16n2/pt_1679-4508-eins-16-02-eRW4074.pdf [ Links ]
18. Melo MM, Cardoso RM, Silva MJS. Reação adversa a medicamento: uma análise comparativa de protocolos utilizados para o tratamento do câncer colorretal. Medicina (Ribeirão Preto, Online). 2017; 50(4): 245-54. Disponível em: http://www.revistas.usp.br/rmrp/article/view/140488/135466 [ Links ]
19. Coolbrandt A, Wildiers H, Aertgeerts B, Elst EV, Laenen A, Casterlé BD, et al. Characteristics and effectiveness of complex nursing interventions aimed at reducing symptom burden in adult patients treated with chemotherapy: A systematic review of randomized controlled trials. International Journal of Nursing Studies. 2014; 51:495-510. Disponível em: https://doi.org/10.1016/j.ijnurstu.2013.08.008 [ Links ]
20. Mollaoglu M, Erdogan G. Effect on symptom control of structured information given to patients receiving chemotherapy. European Journal of Oncology Nursing. 2014; 18(1):78-84. Disponível em: https://doi.org/10.1016/j.ejon.2013.07.006 [ Links ]
21. Brasil. Resolução COFEN n. 569/2018, de 19 de fevereiro de 2018. Aprova o Regulamento Técnico da Atuação dos Profissionais de Enfermagem em Quimioterapia Antineoplásica. Conselho Federal de Enfermagem. Disponível em: http://www.cofen.gov.br/resolucao-cofen-no-0569-2018_60766.html [ Links ]
22. Peiter CC, Caminha MEP, Lanzoni GMM, Erdmann AL. Fatores que interferem no gerenciamento do cuidado ao paciente oncológico em um hospital geral. Revista de Enfermagem da UFSM. 2016; 6(3):404-13. Disponível em: https://periodicos.ufsm.br/reufsm/article/view/21465/pdf [ Links ]
23. Brito KCFV, Souza SR. As necessidade de cuidado do cliente oncológico hospitalizado: aplicação da taxonomia NANDA. Revista de Pesquisa: Cuidado é Fundamental Online. 2017; 9(2):327-32. Disponível em: http://www.seer.unirio.br/index.php/cuidadofundamental/article/view/4138/pdf_1 [ Links ]
Received: September 01, 2020; Accepted: December 21, 2020











 texto en
texto en 


