Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.2 mar./abr. 2006
Physiological bases of bone regeneration II. The remodeling process
Bases fisiológicas de la regeneración ósea II. El proceso de remodelado
Isabel Fernández-Tresguerres Hernández-Gil1,
Miguel Angel Alobera Gracia1,
Mariano del Canto Pingarrón1,
Luis Blanco Jerez2
(1) Profesor Titular Interino. MD. PhD. DDS. Departamento de
Ciencias de la Salud III,
Facultad de Ciencias de la Salud, Universidad Rey Juan
Carlos, Alcorcón
(2) Profesor Titular. MD. PhD. DDS. Departamento de Medicina
y Cirugía Bucofacial,
Facultad de Odontología, Universidad Complutense. Madrid
ABSTRACT
Bone remodeling is the restructuring process of existing bone,
which is in constant resorption and formation. Under normal conditions, this
balanced process allows the renewal of 5 10% of bone volume per year.
At the microscopic level, bone remodeling is produced in
basic multicellular units, where osteoclasts resorb a certain quantity of bone
and osteoblasts form the osteoid matrix and mineralize it to fill the previously
created cavity.
These units contain osteoclasts, macrophages, preosteoblasts
and osteoblasts, and are controlled by a series of factors, both general and
local, allowing normal bone function and maintaining the bone mass. When this
process becomes unbalanced then bone pathology appears, either in excess (osteopetrosis)
or deficit (osteoporosis).
The purpose of this study is to undertake a revision of
current knowledge on the physiological and biological mechanisms of the bone
remodeling process; highlighting the role played by the regulating factors, in
particular that of the growth factors.
Key words: Bone, regeneration, remodeling, resorption, osteogenesis, growth factors.
RESUMEN
El remodelado óseo es un proceso de
reestructuración del hueso existente, que está en constante formación y
reabsorción. Este fenómeno equilibrado permite, en condiciones normales, la
renovación de un 5-10% del hueso total al año. A nivel microscópico el
remodelado óseo se produce en las unidades básicas multicelulares, donde los
osteoclastos reabsorben una cantidad determinada de hueso y los osteoblastos
forman la matriz osteoide y la mineralizan para rellenar la cavidad previamente
creada. En estas unidades hay osteoclastos, macrófagos, preosteoblastos y
osteoblastos y están regidos por una serie de factores, tanto generales como
locales, permitiendo el normal funcionamiento del hueso y el mantenimiento de la
masa ósea. Cuando este proceso se desequilibra aparece la patología ósea,
bien por exceso (osteopetrosis) o por defecto (osteoporosis).
El propósito de este trabajo es realizar una revisión de los conocimientos
actuales sobre los mecanismos bioquímicos y fisiológicos del proceso de
remodelado óseo, resaltando de manera especial el papel de los factores
reguladores del mismo, entre los que destacan los factores de crecimiento.
Palabras clave: Hueso, regeneración, remodelado, reabsorción, osteogénesis, factores de crecimiento.
Introduction
Bone is a dynamic tissue, in constant resorption and formation, permitting the maintenance of bone tissue, the repair of damaged tissue and the homeostasis of the phosphocalcic metabolism. Through this balanced phenomena, known as the remodeling process, about 5% of cortical bone and 20% of trabecular bone is renewed per year. Although cortical bone makes up 75% of the total volume, the metabolic rate is 10 times higher in trabecular bone since the surface area to volume ratio is much greater (trabecular bone surface representing 60% of the total). Therefore, approximately 5 to 10% of total bone is renewed per year. Bone remodeling occurs throughout life, but only up to the third decade is the balance positive. It is precisely in the third decade when the bone mass is at its maximum, this is maintained with small variations until the age of 50. From then on resorption predominates and the bone mass begins to decrease.
At the microscopic level, bone remodeling takes place in small areas of the cortical and the trabecular surface, known as basic multicellular units (BMU). Resorption always precedes formation and in the young skeleton the amount of resorbed bone is similar to the newly formed. For this reason it is referred to as a balanced process, linked in both space and time under normal conditions (1). The average lifespan of each remodeled unit in humans is 2 to 8 months, the greater part of this time being taken up by bone formation. There are 35 million basic multicellular units in the human skeleton, and 3-4 million are activated each year, thus the skeleton is completely renewed every 10 years.
1. Remodeling phases
Bone remodeling can be divided into the following phases (fig 1) (2): quiescent, activation, resorption, formation, mineralization.
1.1 Quiescent phase: said of the bone when at rest. The factors that initiate the remodeling process remain unknown.
1.2 Activation phase: the first phenomena that occurs is the activation of the bone surface prior to resorption, through the retraction of the bone lining cells (elongated mature osteoblasts existing on the endosteal surface) and the digestion of the endosteal membrane by collagenase action. Once exposed, the mineralized surface attracts the circulating osteoclasts coming from the nearby vessels.
1.3 Resorption phase: the osteoclasts then begin to dissolve the mineral matrix and decompose the osteoid matrix. This process is completed by the macrophages and permits the release of the growth factors contained within the matrix, fundamentally transforming growth factor beta (TGF-β), platelet derived growth factor (PDGF), insulin-like growth factor I and II (IGF-I and II).
1.4 Formation phase: simultaneously in the resorbed areas the preosteoblast grouping phenomena is produced, attracted by the growth factors liberated from the matrix which act as chemotactics and in addition stimulate their proliferation (3). The preosteoblasts synthesize a cementing substance upon which the new tissue is attached, and express bone morphogenic proteins (BMP) responsible for differentiation. A few days later, the already differentiated osteoblasts synthesize the osteoid material which fills the perforated areas.
1.5 Mineralization phase: mineralization begins thirty days after deposition of the osteoid, ending at 90 days in the trabecular and at 130 days in the cortical bone.
The quiescent or at rest phase then begins again.
2. Regulatory factors in bone remodeling
The balance between bone resorption and formation is influenced by such interrelated factors as genetic, mechanical, vascular, nutritional, hormonal and local.
2.1 Genetic factors
These are important in determining the maximum bone mass, since between 60 and 80% of this is genetically determined (4). Thus, Negroes have a greater bone mass than Whites, who in turn have a higher mass than Asians. Bone mass is a characteristic transmitted from parents to children, which is why daughters of mothers with osteoporosis are more predisposed to having this condition themselves (5).
2.2 Mechanical factors
Physical activity is essential for the correct development of bone. It is believed that muscular action transmits tension to the bone, which is detected by the osteocyte network within the osseous fluid. These osteocytes produce regulators such as prostaglandins, nitric oxide and IGF-I, which stimulate both their own and the osteoblast activity, increased bone formation. On the other hand, the absence of muscular activity, rest or weightlessness has an adverse effect on bone, accelerating resorption (6).
2.3 Vascular/nerve factors
From studies by Trueta (7) it is known that vascularization is fundamental for normal bone development, supplying blood cells, oxygen, minerals, ions, glucose, hormones and growth factors. Vascularization constitutes the first phase in ossification: the blood vessels invade the cartilage and later produce resorption via the osteoclasts originating from the nearby vessels. In the same way, vascular neoformation is the first event in the repair of fractures or bone regeneration, since the supply of oxygen is fundamental to the production of the restitutio ad integrum rather than fibrous tissue. Ham described this phenomenon in 1952 (8), observing that the osteocytes die when they are at some distance from a capillary vessel (the maximum distance being 0.1 mm).
Innervation is necessary for normal bone physiology. The bone is innervated by the autonomous nervous system and by sensorial nerve fibers. Autonomous fibers have been found in periosteum, endosteum, cortical bone and associated with the blood vessels of the Volkmann conduit, and likewise neuropeptides and their receptors in bone. Examples of the importance of innervation in bone physiology are found in osteopenia and the bone fragility present in patients with neurological disorders, and also in the decreased bone density in de-nerved mandibles.
2.4 Nutritional factors
This factor is interesting because it can be modified. A minimum amount of calcium is needed for mineralization, which the majority of authors put at 1,200 mg per day to the age of 25, not less than 1g per day from 25 to 45, and following menopause should be at least 1,500 mg per day. Likewise, it is known that toxic habits such as smoking, caffeine, alcohol and excess salt constitute risk factors for osteopenia.
2.5 Hormonal factors
Normal development of the skeleton is conditioned by the correct functioning of the endocrine system, fundamentally of the growth hormone (GH) and the calciotropic hormones (parathyroid hormone, calcitonin, and metabolites of vitamin D). Hormones are systemic messengers that act at a distance from the site of production (endocrine effect), but also regulate the synthesis and action of local factors, which intervene directly in the cellular metabolism (autocrine and paracrine effects).
The most important hormones in bone physiology are:
2.5.1 Thyroid hormones: possess two opposing actions on bone. In the first place, they stimulate the synthesis of the osteoid matrix by the osteoblasts and its mineralization, favoring the synthesis of IGF-I. For this reason, in congenital hypothyroidism (cretinism) short stature is produced by the alteration in bone formation. In the second place, a contrary effect is produced, stimulating resorption with the increase in number and function of the osteoclasts. The clinical manifestation of this effect is the appearance of bone loss in hyperthyroidism (9).
2.5.2 Parathyroid hormone (PTH): controls the homeostasis of calcium by direct action on the bone and the kidneys, and indirectly on the intestine. Produced in the parathyroid glands in response to hypocalcemia, stimulating bone resorption it is the preeminent hypercalcemic hormone. However, in recent years a stimulating role in bone formation, through the synthesis of IGF-I and TGF-β, has been discovered (10). This dual effect of resorption and formation can be explained because the continual supply of PTH would stimulate bone resorption through the synthesis of a factor favoring osteoclastogenesis (RANKL) on the part of the osteoblastic cells, while at intermittent doses it would stimulate the formation of bone, associated with an increase of the above mentioned growth factors and with a decrease in the apoptosis of the osteoblasts.
2.5.3 Calcitonin: is produced in the C, or parafollicular, cells of the thyroid, it is an inhibitor of bone resorption, reducing the number and activity of the osteoclasts. However, this is a transitory action, since the osteoclasts seem to become impermeable to calcitonin within a few days (11).
2.5.4 1.25(OH)2 vitamin D3 or calcitriol: a steroid hormone which favors the intestinal absorption of calcium and phosphate, and therefore bone mineralization. It is necessary for normal growth of the skeleton. Some authors believe it may be produced by lymphocytic or monocytic bone cells, playing an important role as a local regulator of osteoclast differentiation (12).
2.5.5 Androgens: have an anabolic effect on bone through the stimulation of the osteoblast receptors. Likewise, they act as mediators of the peak GH in puberty. While androgen deficiency is associated with lower bone density, the administration of testosterone in young people before the closure of the epiphyses increases bone mass. In the same way, women with an excess of androgens present higher bone densities.
2.5.6 Estrogens: are essential for the closure of the growth platesand have been discovered to play an important role in the development of the skeleton, both masculine and feminine, during adolescence. Estrogens have a dual effect on bone metabolism: on the one hand they favor bone formation, increasing the number and function of the osteoblasts, and on the other they reduce resorption. Estrogen receptors have been described in human osteoblasts, osteocytes and osteoclasts. Recent investigations have found that estrogens can increase the levels of osteoprotegerin (OPG), a protein produced by osteoblasts that inhibits resorption (13), so they may play an important role in the regulation of osteoclastogenesis. For this reason estrogen deficiency during menopause constitutes the most important pathogenic factor in bone loss associated with osteoporosis.
2.5.7 Progesterone: also has an anabolic effect on bone, either directly, through the osteoblasts which possess hormone receptors, or indirectly, through competition for the osteoblastic receptors of the glucocorticoids.
2.5.8 Insulin: stimulates matrix synthesis both directly and indirectly, increasing the hepatic synthesis of IGF-I (insulin-like growth factor).
2.5.9 Glucocorticoids: at high doses they have a catabolic effect on bone, since they inhibit the synthesis of IGF-I by the osteoblasts, and directly suppress BMP-2 and Cbfa1, critical factors in osteoblastogenesis (14). However, recent studies have demonstrated that at physiological doses they have an osteogenic capacity favoring osteoblastic differentiation (15).
2.5.10 Growth Hormone: acts both directly and indirectly on bone. Growth hormone acts directly on the osteoblasts with hormone receptors, stimulating their activity, thus increasing the synthesis of collagen, osteocalcin and alkaline phosphate. The indirect action is produced through an increase in synthesis of IGF-I and II by the osteoblasts. These factors stimulate the proliferation and differentiation of the osteoblasts, increasing their number and function. GH has been considered as a local growth factor, since it is not only synthesized in the adenohypophysis, but also in almost all the cells of the organism, including osteoblasts (16), having both an autocrine and paracrine effect in addition to the endocrine.
2.6 Local factors
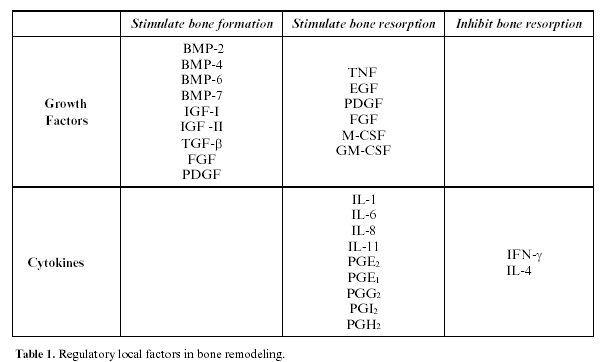
Bone remodeling is also regulated by local factors, among which principally growth factors and cytokines, and recently the bone matrix proteins have been implicated as modulators of other local factors (table 1). Bone cells also play an important role in the production of prostaglandins and nitric oxide, as well as cytokines and growth factors.
2.6.1 Growth factors
These are polypeptides produced by the bone cells themselves or in extra-osseous tissue, and act as modulators of the cellular functions, fundamentally growth, differentiation, and proliferation (table 1).
IGF-I and II (insulin-like growth factor I and II): these are polypeptides similar to insulin; they are synthesized by the liver and osteoblasts, and found in high concentrations in the osteoid matrix (17). They increase the number and function of the osteoblasts, stimulating collagen synthesis. They circulate linked to IGF-binding proteins (IGFBP), which in turn can exercise stimulatory or inhibitory effects on bone. IGF synthesis is regulated by hormones and local growth factors; thus GH, estrogens and progesterone increase their production, while the glucocorticoids inhibit it. They also mediate in the osteoblast-osteoclast interaction and actively participate in bone remodeling (18). IGF-II is the most abundant factor in the bone matrix, it is important during embryogenesis, but its effects on the fully developed skeleton are as yet unknown (19).
Transforming Growth Factor-β(TGF-β): is a superfamily of proteins highly abundant in bone tissue (second after IGF). They are latently present in the matrix and activate during osteoclastic resorption. TGF-β is a potent stimulator of bone formation, promoting osteoblastic differentiation and the synthesis of the osteoid matrix, and inhibiting the synthesis of the proteases (especially the matrix metalloproteinase (MMP), an enzyme which degrades it).
Likewise, TGF-β inhibits resorption on reducing the formation and differentiation of the osteoclasts, as well as mature osteoclast activity and stimulating their apoptosis (20).
However, in addition to these functions, it has been discovered to inhibit epithelial proliferation and mediate in the anabolic effect of the androgens.
Bone Morphogenetic Proteins (BMP): are included in the TGF-β family. They form a group of 15 proteins able to achieve the transformation of connective tissue into bone tissue, for which they are considered osteoinductive. Likewise, they are able to stimulate the differentiation of the stem cells towards different cell lines (adipose tissue, cartilage and bone). They are highly abundant in bone tissue, and during embryogenesis participate in the formation of bone and cartilage. They are currently considered to be the most powerful factors in osteoblastic differentiation (21). Canalis et al. (22) believe that besides stimulating osteogenesis, they inhibit osteoclastogenesis.
Platelet-Derived Growth Factor (PDGF): on the one hand it stimulates proteic synthesis brought about the osteoblasts (23), and on the other, favors bone resorption. Other effects are the proliferation of fibroblasts and smooth muscle cells, neovascularization, and collagen synthesis, therefore favoring scarring.
Fibroblastic Growth Factor (FGF): has an anabolic effect on bone, as it is a mitogen of osteoblasts, vascular endothelial cells, and fibroblasts. As a practical example of the effect of FGF it is known that mutations in its receptors produce alterations in the craniofacial skeleton, such as achondroplasia, Aperts syndrome and Crouzons syndrome, among others (24).
Epidermal Growth Factor (EGF): is a powerful mitogen of cells of mesodermic or ectodermic origin.
It is synthesized in many tissues and could therefore be involved in diverse, as yet unexplained, biological functions. With respect to bone, it could have a dual formative and destructive action, although the latter is the most well known.
Vascular Endothelial Growth Factor (VEGF): induces angiogenesis and vascular endothelial proliferation. It produces vasodilation and an increase in vascular permeability. It is produced in hypoxia and is currently considered one of the key factors in the first phases of fracture repair and bone regeneration, as well as in tumor growth.
Granulocyte/Macrophage-Colony Stimulating Factor (GM-CSF): is important in osteoclastogenesis and may play a role in the pathogeny of osteopetrosis.
Macrophage-Colony Stimulating Factor (M-CSF): is produced by osteoblasts and medullar stromal cells, it is an essential factor in the first phases of osteoclastogenesis being required for the formation of giant multinucleate cells, but has no effect on osteoclastic activity.
Tumor Necrosis Factor (TNF): in vitro stimulates resorption and has been related with bone loss in arthritis and periodontal disease.
2.6.2 Matrix Proteins
The matrix proteins have recently been discovered to act as growth factor modulators (25). It should be taken into account that matrix proteins are found in concentrations a thousand times higher than growth factors, and could therefore play a more important role in the regulation of the different cell functions (26).
Furthermore, these matrix proteins also participate in regulation of the differentiation of the cells contained within the matrix. For example, collagen I is one of the earliest markers which regulates the osteoprogenitor cells, and alkaline phosphatase is a surface protein that could participate in the regulation of the proliferation, migration and differentiation of the osteoblastic cells.
2.6.3 Cytokines
These are polypeptides synthesized in the lymphocytic and monocytic cells and play an important role in multiple cellular functions, such as the immunological response, inflammation and hematopoiesis, having both an autocrine and paracrine effect.
The following are important in bone:
- Interleukin 1 (IL-1): directly stimulates osteoclastic resorption, increasing the proliferation and differentiation of the pre-osteoblasts as well as the osteoclastic activity and inhibiting the apoptosis of the osteoclasts (2). In reality, they are 3 different related molecules: IL-1α, IL-1β and the IL-1 receptor antagonist, this last being the inhibitor of the first two. Its action on resorption, through the synthesis of prostaglandins, is both direct and indirect.
- Interleukin 6 (IL-6): stimulates bone resorption and appears to be implicated in the pathogenesis of Pagets disease (27). It is believed to play an important role in the initial stages of osteoclastogenesis, and is produced in response to PTH, IL-1 and 1.25(OH)2D3.
- Interleukin 11 (IL-11): recently discovered, it is produced in bone marrow and induces osteoclastogenesis.
- Prostaglandins (PG): in vitro stimulate bone resorption, fundamentally PGE2, but also PGE1, PGG2, PGI2 and PGH2 (28). Studies in vivo, measuring the prostaglandin levels in the crevicular liquid, have demonstrated its participation in the bone destruction that takes place in periodontal disease (29).
3. Biochemical markers of bone metabolism
The biochemical markers of bone metabolism are interesting from the clinical point of view in evaluating the remodeling process. Thus, there are markers of bone formation, such as alkaline phosphatase, osteocalcin and procollagen type I (PICP); and markers of resorption, such as hydroxyprolinuria and tartrate resistant phosphatase acid. Of the 11 most frequently used biochemical markers to measure bone resorption and formation, 9 are extracellular matrix proteins (25). The markers of osteo-formation are products of the osteoblasts at their different stages of differentiation (30) (table 2).
![]() Correspondence
Correspondence
Dra. Isabel Fernández-Tresguerres Hernández-Gil
Facultad de Ciencias de la Salud, Avda de Atenas s/n
Alcorcón, 28922 Madrid.
Teléfono: 914888941.
E-mail: isatresguerres@yahoo.es
Received: 2-08-2004
Accepted: 14-08-2005
References
1. Parfitt AM. The coupling of bone formation to bone resorption: A critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 1982;4:1-6. [ Links ]
2. Compston JE. Sex steroids and bone. Physiol Rev 2001;81:419-47. [ Links ]
3. Lind M, Deleuran B, Thestrup-Pedersen K, Soballe K, Eriksen EF, Bunger C. Chemotaxis of human osteoblasts. Effects of osteotropic growth factors. APMIS 1995;103:140-6. [ Links ]
4. Grant SFA, Ralston SH. Genes and osteoporosis. Endocrinology 1997;8:232-9. [ Links ]
5. Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrock PN, Ebery S. Genetic determinants of bone mass in adults. J Clin Invest 1987;80:706-10. [ Links ]
6. Morey ER, Baylink JJ. Inhibition of bone formation during space flight. Science 1978;19:172-6. [ Links ]
7. Trueta J. The role of blood vessels in osteogenesis. J Bone Joint Surg Br 1963;45:402. [ Links ]
8. Ham AW. Some histophysiological problems peculiar to calcified tissue. J Bone Joint Surg Am 1952;34:701. [ Links ]
9. Jódar Gimeno E, Muñoz-Torres M, Escobar-Jiménez F, Quesada Charneco M, Luna del Castillo JD, Olea N. Identification of metabolic bone disease in patients with endogenous hypertiroidism: Role of biological markers of bone turn-over. Calcif Tissue Int 1997;61:370-6. [ Links ]
10. Canalis E, McCarthy TL, Centrella M. The role of growth factors in skeletal remodeling. Endocrinol Metab Clin North Am 1989;18:903-18. [ Links ]
11. Prieto S. Control del metabolismo del calcio, fósforo y magnesio. En: Tresguerres JAF, ed. Fisiología Humana, 2ª edición. Madrid: McGraw-Hill-Interamericana; 1999.p.979-93. [ Links ]
12. Raisz LG. Bone cell biology: New approaches and unanswered questions. J Bone Miner Res 1993;8:457-65. [ Links ]
13. Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expresion and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999;140:4367-70. [ Links ]
14. Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000;21:115-37. [ Links ]
15. Lukert BP, Kream BE. Clinical and basic aspects of glucocorticoid action in bone. En: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, California: Academic Press;1996.p.533-48. [ Links ]
16. Harvey S, Hull KL. Growth hormone: A paracrine growth factor? Endocrine 1998;7:267-79. [ Links ]
17. Cohick WS, Clemmons DR. The insulin-like growth factors. Ann Rev Physiol 1993; 55:131-53. [ Links ]
18. Hill PA, Reynolds JJ, Meikle MC. Osteoblasts mediate insulin-like gowth factor-I and -II stimulation of osteoclasts formation and function. Endocrinology 1995;136:124-31. [ Links ]
19. Mohan S, Baylink DJ. Bone growth factors. Clin Orthop 1991;263:30-48. [ Links ]
20. Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res 1993;8:565-72. [ Links ]
21. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by Bone Morphogenetic Proteins, Hedgehogs, and Cbfa1. Endocr Rev 2000;21:393-411. [ Links ]
22. Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 2003;24:218-35. [ Links ]
23. Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effects of platelet-derived growth factor on tibial osteotomies in rabbits. Bone 1994;15:203-8. [ Links ]
24. Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 2003;316:23-32. [ Links ]
25. Young MF. Bone matrix proteins: more than markers. Calcif Tissue Int 2003;72:2-4. [ Links ]
26. Horowitz M. Matrix proteins versus cytokines in the regulation of osteoblasts function and bone formation. Calcif Tissue Int 2003;72:5-7. [ Links ]
27. Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A. Interleukin-6: A potential autocrine/paracrine agent in Paget´s disease of bone. J Clin Invest 1992;89:46-52. [ Links ]
28. Kawaguchi H, Pilbean CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop 1995;313:36-46. [ Links ]
29. Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expresion. J Periodontol 1993;64:432-44. [ Links ]
30. Schonau E, Rauch F. Markers of bone and collagen metabolism. Problems and perspectives in Pediatrics. Horm Res 1997;48:50-9. [ Links ]











 texto en
texto en