Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.4 jul. 2006
Follicular adenomatoid odontogenic tumor: immunohistochemical study
Tumor odontogenico adenomatoide folicular: Estudio inmunohistoquimico
Francisco José Vera Sempere1, María José Artes Martínez1, Beatriz Vera Sirera1, Jaime Bonet Marco2
1Department of Pathology
2Department of Cirugía Maxilofacial Infantil. Hospital Universitario La Fe, Universidad de Valencia
ABSTRACT
Adenomatoid odontogenic tumor (AOT) is an uncommon benign odontogenic lesion that affects young
patients, with female predominance, mainly in second decade, showing a
radiolucent unilocular image associated with an unerupted tooth, usually a
canine.
In spite of previous and confusing denominations, such as adenoameloblastoma or adenomatoid ameloblastic tumor,
AOT is a benign tumor with a very low rate of recurrence, that show a peculiar
morphological picture (basaloid appearance with glandular-like structures,
calcifying areas, and amiloid-like material) that allow its histopathological
recognition.
We present a clinicopathological analysis of a case of
follicular AOT affecting the mandible in a 9 years-old female patient associated
with unerupted lower left canine. Immunohistochemical study showed some data previously
unrecognised. All cellular types that composed AOT showed nuclear positivity for
p63 indicating a basal characterization in the different cellular components.
According to its benign character and low potential for recurrence, AOT revealed
a scant proliferative activity (2-3% nuclei showed Ki-67 positivity) limited to
some epithelial nodules (AE1-3 +) of fusiform appearance. Absence of reactivity
for hormonal receptors (RE and RPg) excluded a possible hormonodependence in AOT
that could explain the observed female predominance.
Key words: Adenomatoid odontogenic tumor, immunohistochemistry, p63, Ki67.
RESUMEN
El tumor odontogénico adenomatoide (TOA) es una infrecuente
lesión odontogénica benigna, que aparece en pacientes jóvenes, generalmente
mujeres en la segunda década de la vida, a menudo como una lesión radiolúcida de
aspecto quístico unilocular, en asociación a un diente, usualmente canino, no
erupcionado.
A pesar de haberse denominado también adenoameloblastoma o
tumor ameloblástico adenomatoide, el TOA es una lesión benigna con una muy baja
tendencia a la recidiva, mostrando una morfología muy peculiar (apariencia
basaloide con estructuras glanduliformes, calcificaciones esferulares, presencia
de material amiloide) que facilitan su reconocimiento histológico.
Se presenta un análisis clínico-patológico de un TOA de tipo
folicular de maxilar inferior, en asociación a la inclusión de un canino
inferior izquierdo, en una paciente pediátrica de 9 años. El estudio
inmunohistoquímico realizado muestra algunos datos previamente no referidos. A
pesar de existir distintos tipos celulares en el TOA se observo una universal
inmunorreactividad para p63, demostrando el carácter basal de los distintos
elementos que lo constituyen. En concordancia con su benignidad y con su baja
tasa de recidiva existe una escasa actividad proliferativa (2-3% de núcleos
marcados por el antígeno Ki-67), estando la proliferación reducida a pequeños
nódulos de células epiteliales (AE1-3 +) de núcleos elongados o fusiformes; de
otra parte la mayor incidencia en pacientes de sexo femenino no puede ser
explicada en base a la existencia de una hormonodependecia tumoral dada la
ausencia de expresión de receptores hormonales (RE y RPg).
Palabras clave: Tumor odontogénico adenomatoide, inmunohistoquímica, p63, Ki67.
Introduction
Adenomatoid odontogenic tumor (AOT) is an uncommon, benign epithelial lesion of odontogenic origin, representing approximately 3% of all odontogenic tumors (1). The tumor occurs more frequently in females with a ratio of 2:1, and appears most often in the second decade of life.
Described for the first time by Dreiblat in 1907 as an adenoameloblastoma, and among others has also been named ameloblastic adenomatoid tumor. In 1969 Philipsen and Birn (2) proposed the term AOT, indicating that it did not constitute a variety of ameloblastoma, and was accepted as such in the first WHO classification of odontogenic tumors established in 1971 (3). The term AOT is without doubt the most appropriate, in that these tumors are clearly benign and, in contrast to the ameloblastoma, present a very low recurrence (4), making it unnecessary to carry out extensive and aggressive surgery; a simple curettage in conjunction with the extirpation of the associated tooth being the indicated treatment.
AOT is a slow-growing tumor with three clinical subtypes (4), all with identical histology: follicular type (in 73% of AOT), centrally located, with a radiolucent unilocular cystic area associated with an unerupted or impacted tooth (usually canine), simulating the image of a dentigerous cyst; the extrafollicular variant (24%), likewise with a central location, but unrelated to any dental structure, and may be confused with periapical cysts and other cystic or tumoral lesions of the maxilla (5); finally the peripheral form is the most infrequent (3%), it affects the gingival mucosa, and is often preoperatively classified as a fibrous epulis or gingival fibroma.
Histologically, AOT is highly unusual, as it presents a variety of cellular patterns: in some areas basophilic cells with fusiform or elongated nuclei, forming whorled nodules appear; while in other areas, clearly polarized cylindrical cells, forming glandular or duct-like structures are present. Among the epithelial cells, variable quantities of amorphous eosinophilic material are often found, referred to by some authors as eosinophilic drops. Spherical calcifications may occasionally appear, interpreted as the formation of abortive enamel, and which can confer a degree of radiological opacity to the tumor; likewise groups of cells resembling the structure of calcifying epithelial odontogenic tumor (6), in which eosinophilic extracellular amyloid-like material can form (7).
In this study, we present a clinicopathological observation of the follicular variety of this infrequent benign tumor, affecting the anterior area of the mandible, associated with an impacted lower left canine in a 9-year-old pediatric patient. We demonstrate the results of the immunohistochemical study, which provides new data on the immuno-morphological characterization of this tumor and its limited proliferative capacity.
Clinical case
A nine-year-old pediatric patient, with no clinical history of interest, was referred to the Infant Maxillofacial Surgical Unit presenting dental impaction.. The radiological study revealed impaction of the lower left canine, with a radiolucent area around tooth 33, the patient was admitted for surgical correction with a preoperative diagnosis of follicular cyst. Surgery consisted of extirpation of the follicular cyst with curettage of the bone cavity and extraction of the unerupted canine, followed by osseous filling with Bio-Oss, and having a satisfactory post-surgical evolution.
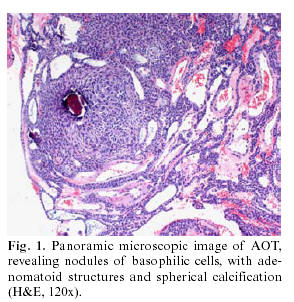
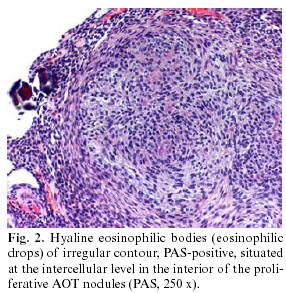
The surgical specimen resulting from the curettage consisted of various irregular, bland, grayish fragments, which when grouped together measured 0.8 x 0.7 cm, plus the extracted canine (0.9 x 0.5 cm). The histological study demonstrated an unaltered dental structure, accompanied by fragments of fibrous tissue, with numerous solid nests of odontogenic epithelial elements in the interior arranged in compact micronodular formation with a whorled cellular arrangement (Fig. 1). The elements forming these structures were of basaloid morphology, with oval or fusiform monomorphous nuclei, somewhat hyperchromatic, although with no evidence of divisional activity. Glandular formations appeared mixed with these structures, sometimes of tubular appearance, with a covering of homogenous, cylindrical cells, with nuclei often polarized towards the base. At the intercellular level there were small, dispersed, calcified basophilic spherules, as well as areas of irregular hyaline and amorphous deposits, PAS + diastase resistant (Fig. 2), positive to Congo red although with no green refraction observed in polarized light. The resulting diagnosis was that of follicular adenomatoid odontogenic tumor associated with an unerupted canine.
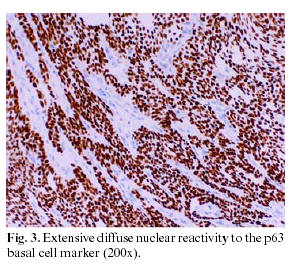
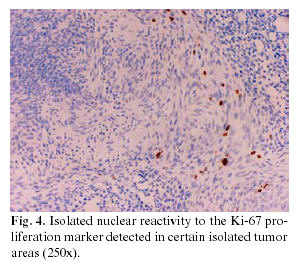
An immunohistochemical study was carried out on the soft tissue resulting from the extirpation, which in the first place revealed reactivity to proliferative elements, both in the nodular and adenomatoid areas, against a cocktail of AE1-3 keratins. Likewise, nuclear positivity for the p63 protein (progenitor or basal cell marker) was observed, this nuclear reactivity being present in both the glandular areas and in the whorled nests of fusiform cells (Fig. 3). The Ki-67 proliferation marker (Fig. 4) marked only 2-3% of the cells constituting the tumor, with the positivity often grouped to nodules of fusiform cells. The detection of melanic HMB45 and Melan-A differentiation markers was negative, as it was for the estrogen and progesterone receptors (ER) and (PgR), and for beta-2-microglobulin.
Discussion
The present case study illustrates the clinicopathological characteristics of the follicular variety of AOT, a tumor, which as occurred in this case is often preoperatively interpreted as a follicular odontogenic cyst (8), given the association with an unerupted or impacted tooth.
The first point of interest with this tumor is to make a correct anatomicopathological identification and not to confuse it with other, more aggressive, forms of odontogenic tumors, which can be further complicated by the use of inappropriate terms such as adenoameloblastoma. Given its benign behavior, slow growth and clear delimitation, as well as its low tendency to recur, the treatment of choice is enucleation and simple curettage, although in exceptional cases of large tumors or risk of bone fracture, partial resection en bloc of the anterior mandible or maxilla has been indicated (9). Additionally, the use of lyophilized bone and guided tissue regeneration has been recommended in cases where surgical extirpation has left a large exposed osseous cavity (10).
Structurally, AOT is a well-circumscribed lesion, derived from odontogenic epithelium, which, at least in the follicular variety as described here, usually establishes itself around the corona of unerupted, anterior teeth in young patients, being constituted of whorled nests of epithelium together with areas of glandular or ductal patterns intermixed with occasional spherical calcifications (1).
However, within this structural configuration, some unusual morphological aspects of uncertain origin do appear, which although identified histologically, their histogenesis and significance remain to be clarified. The first of these unusual characteristics is the presence of amorphous eosinophilic material, PAS positive diastase-resistant, with some staining characteristics similar to amyloid (11), and positivity to Congo red, but without refraction in polarized light, both aspects confirmed in our observation and whose origin is interpreted by some authors as deposits of basal membrane type material (12). The absence of refraction in polarized light of the Congo red-positive amyloid material in our observation, contrasts with the data recently published by León et al. (7) who indicated a positive refraction in 25.6% of the AOT studied, although the presence of refractive images in maltese cross indicated in this study is not specific nor characteristic of amyloid. Ultrastructurally, this material can present a granular or fibrillar configuration, but without the characteristic submicroscopic fibrillae of amyloid (13) with some authors also finding histochemical characteristics concordant with enamel matrix material (14).
Another potential morphological factor, not found in our case (which demonstrated negativity for HMB45 and Melan-A), is the possible presence of melanocytes and/or melanic pigmentation (15) in the interior of the tumor nests, a morphological aspect, which aside from having no clinical or prognostic significance, has also been related with a neurocristopathic influence in the development of odontogenic tissue. Finally, the absence of nuclear reactivity for hormonal receptors (RE and RPg) observed in our study, does not allow us to establish any endocrine influence in the origin of these tumors, nor to explain its higher frequency in females.
The slow growth of AOT, its benign character and its low tendency to recur are clearly related to the low cellular proliferation observed on carrying out immunostaining for the Ki67 antigen. Only 2-3% of the components of the tumor demonstrated reactivity for Ki67, a percentage similar to that recently indicated by León et al. (7), this positivity being centered in the whorled nodules of fusiform cells that seem to establish themselves as the sole centers of the tumor proliferation. A second point of interest is the marked and diffuse positivity for AE-1-3 associated with a universal nuclear reactivity of the p63 antigen throughout practically all the cells forming this tumor. From the immunohistochemical point of view, this last aspect confirms the basal and/or progenitor (p63+) character of epithelial (AE1-3+) cells of the elements that make up this benign tumor of low grade proliferation, although possessing a wide phenotypic morphological variety (7).
Much of the above data (low cellular proliferative capacity, basal character confirmed by the universal reactivity of p63 of all the proliferating cells, the presence of spherical calcification as forms of abortive enamel, or the inadequate formation of dentinoid tissue, likewise the existence of mesenchymal inductive changes with production of amyloid-like material) would seem to reinforce the idea of the hamartomas character of this infrequent form of benign, odontogenic tumor, as previously indicated by other authors (1,5) on indicating that this is probably not a true neoplastic growth.
![]() Correspondence:
Correspondence:
Prof. Francisco José Vera Sempere
C/ Daoiz y Velarde 8, pta. 14
46021 Valencia
E-mail: vera_fra@gva.es
Received:22-10-2005
Accepted: 4-03-2006
References
1. Cossío I, Rodríguez-Armijo Sánchez L, García Calderon M, Gutiérrez Pérez JL, González Cámpora R. Tumor odontogénico adenomatoide de maxilar superior. Med Oral 1997;2:168-71. [ Links ]
2. Philipsen HP, Birn H. The adenomatoid odontogenic tumour. Ameloblastic adenomatoid tumour or adenoameloblastoma. Acta Pathol Microbiol Scand 1969;75:375-98. [ Links ]
3. Pindborg JJ, Kramer IRH. WHO histological typing of odontogenic tumors, jaw cysts and allied lesions. Geneva. 1971. [ Links ]
4. Philipsen HP, Reichart PA, Zhang KH, Nijai AH, Yu QX. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med 1991;20:149-58. [ Links ]
5. Philipsen HP, Srisuwan T, Reichart PA. Adenomatoid odontogenic tumor mimicking a periapical (radicular) cyst: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:246-8. [ Links ]
6. Mosqueda-Taylor A. Carlos-Bregni R, Ledesma-Montes C, Fillipi RZ, de Almeida OP, Vargas PA. Calcifying epithelial odontogenic tumor-like areas are common findings in adenomatoid odontogenic tumors and not a specific entity. Oral Oncol 2005;41:214-5. [ Links ]
7. Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, de Almeida OP, Mosqueda-Taylor A, et al. Clinicopathological and immunohistochemical study of 39 cases of adenomatoid odontogenic tumour: a multicentric study. Oral Oncol 2005;41:835-42. [ Links ]
8. Bravo M, White D, Miles L, Cotton R. Adenomatoid odontogenic tumor mimicking a dentigerous cyst. Int J Pediatr Otorhinolaryngol 2005;69:1685-8. [ Links ]
9. Nomura M, Tamimoto K, Takata T, Shimomato T. Mandibular adenomatoid odontogenic: tumor with unusual clinicopathologic features. J Oral Maxillofac Surg 1992;50:282-5. [ Links ]
10. Vitkus R, Meltzer JA. Repair of a defect following the removal of a maxillary adenomatoid odontogenic tumor using guided tissue regeneration. A case report. J Periodontol 1996;67:46-50. [ Links ]
11. El-Labban NG. The nature of eosinophilic and laminated masses in the adenomatoid odontogenic tumor: a histochemical and ultrastructural study. J Oral Pathol Med 1992;21:75-81. [ Links ]
12. Takahashi H, Fujita S, Shibata Y, Yagamuchi A. Adenomatoid odontogenic tumour: immunohistochemical demonstration of transferrin, ferritin and alpha-one-antitrypsin. J Oral Pathol Med 2001;30:237-44. [ Links ]
13. Schlosnagle DC, Someren A. The ultrastructure of the adenomatoid odontogenic tumor. Oral Surg Oral Med Oral Pathol 1981;52:154-61. [ Links ]
14. Mori M, Makino M, Imai K. The histochemical nature of homogeneous amorphous materials in odontogenic epithelial tumors. J Oral Surg 1980;38:96-102. [ Links ]
15. Warter A, George-Dilombi G, Chazal M, Ango A. Melanin in a dentigerous cyst and associated adenomatoid odontogenic tumor. Cancer 1990;66:786-8. [ Links ]











 texto en
texto en