Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.6 nov./dic. 2006
Variations of interleukin-6 after surgical removal of lower third molars
Variaciones de la interleuquina-6 tras la cirugía del tercer molar inferior
Carmen López Carriches1, José Mª Martínez-González2, Manuel Donado Rodríguez3
(1) Associate Professor of Adults Integrated Odontology, Universidad Europea
(2) Professor of Maxillofacial Surgery, Universidad Complutense
(3) Full Professor of Maxillofacial and Oral Surgical Pathology, Universidad Complutense. Madrid.
ABSTRACT
Objectives: To determine if there is a release of IL-6 after surgical removal of lower third molars and to compare the amount of IL-6 in patients treated with NSAID and in those treated with glucocorticoids.
Study Design: Prospective study on 73 patients who attended the Oral Surgery Unit (Department of Medicine and Oral Surgery) in the Faculty of Odontology of the Universidad Complutense de Madrid for the surgical removal of their lower third molars. These patients were separated into two groups: the diclofenac group and the methylprednisolone group. A record card was completed with preoperative and postoperative epidemiological and clinic data. Samples of gingival crevicular fluid were collected in order to assess the release of interleukin-6 after surgery. In order to make a broad study of data, the BMDP program was used for statistical analysis.
Results: Levels of IL-6 were higher after surgical extraction of lower third molars and remained high until the seventh day after. Levels were higher in the diclofenac group 24 hours after surgery, the difference was significant (0.008).
Conclusions: IL-6 is higher after surgical extraction of lower third molars, behaving differently in each of the groups.
Key words: Third molar surgery, cytokines, interleukin-6, methylprednisolone, diclofenac.
RESUMEN
Objetivos: Determinar si tras la cirugía del tercer molar inferior se produce una liberación de interleuquina-6 (IL-6) y comparar la cantidad de IL-6 en pacientes que tomaron AINES y en aquellos que tomaron glucocorticoides.
Diseño del estudio: Estudio prospectivo sobre 73 pacientes sometidos a la extracción quirúrgica de los terceros molares inferiores. Fueron divididos en dos grupos: De diclofenaco y de metilprednisolona. Se recogieron muestras de fluído crevicular gingival para valorar la liberación de interleuquina-6 tras la cirugía. Se usó el programa estadístico BMDP para hacer un amplio tratamiento de los datos.
Resultados: Los niveles de IL-6 se elevaron tras la cirugía del tercer molar inferior permaneciendo elevados al séptimo día del postoperatorio, elevándose más a las 24 horas en el grupo de diclofenaco siendo esta diferencia significativa (0,008).
Conclusiones: La IL-6 se eleva tras la cirugía del tercer molar inferior, presentando diferente comportamiento en los dos grupos de estudio.
Palabras clave: Cirugía del tercer molar, citoquinas, interleuquina-6, metilprednisolona, diclofenaco.
Introduction
In the last few years, a lot of research has been carried out on the utility of certain cytokines, such as interleukin-1 and interleukin-6, as activity markers in periodontitis. However, to our knowledge, no study has been published on the utility of these substances in the surgical extraction of lower third molars (1,2). IL-6 plays an important role in inflammation, together with IL-1 and the Tumor Necrosis Factor (TNF).
In the maxillofacial area, Miyawaki et al. (3) have proved that the level of IL-6 in plasma increases after different operations (cystectomy, benign tumor extirpation, etc.) and therefore, we assume that a large amount of cytokines (IL-6 among them) are released locally after surgical removal of lower third molars, and that to a great extent these are responsible for postoperative complications.
Cruickshank et al. (4) studied the response of IL-6 in patients who had undergone different types of operations (minor surgery, cholecystectomy, hip surgery, colorectal surgery and major vascular surgery), finding that levels of IL-6 were related to the duration of surgery. The authors concluded that IL-6 is a sensitive and early marker of tissue damage.
The concentration of IL-6 may also change depending on the antiinflammatory used during the postoperative period, since there are studies which demonstrate that glucocorticoids inhibit the production of this cytokine (5).
The objectives of this study were to determine if there is a release of IL-6 after surgical removal of lower third molars and to compare the amount of IL-6 in patients treated with NSAID and in those treated with glucocorticoids.
Patients and method
The study was carried out on 73 patients who attended the Oral Surgery Unit (Department of Medicine and Oral Surgery) in the Faculty of Odontology of the Universidad Complutense de Madrid for the surgical removal of their lower third molars. As laboratory material we used paper points (Periopaper strip®. Proflow Incorporated. New York. USA), Periotron® 6000 (Proflow Incorporated. New York. USA), tubes (Microspin filters®-Teknocroma-) and IL-6 kit (ICN Pharmaceuticals, Inc. California). The following equipment was used: a freezer to maintain the samples at –80ºC, microsyringe (Hamilton Bonaduz A. G. Switzerland), micropipettes, cup vibrator (IKA-Works Inc. ®USA) with speeds between 200-2500/min, automatic washer (Easy washer EAW 8/12®), reader (Easy reader EAR 400®) and printer and centrifuge. (Selecta®).
Inclusion criteria were as follows: Patients over 18 whose lower third molars required extraction. Informed consent to participate in the study was signed. Without systemic pathology and without clinical symptoms in the third molar.
Exclusion criteria were: patients with systemic pathology, pregnant or breast-feeding women, or patients with symptomology in the third molar or who had taken anti-inflammatories within the previous 7 days.
Two groups were formed at random: in group A, 36 patients took oral diclofenac sodium (Voltaren®) at doses of 50 mg every 8 hours for the first three days after surgery; and in group B, 37 patients took oral methylprednisolone (Urbason®) at doses of 4 mg every 8 hours also for three days. All patients received antibiotic treatment with oral amoxicillin at doses of 750 mg every 8 hours during 7 days after surgery. Those who were still in pain were able to request the oral administration of a rescue drug, magnesium metamizol (Nolotil®), at doses of 575 mg every 6 or 8 hours for pain relief.
Sterile paper points (Periopaper Strip®) were used to collect five samples of gingival crevicular fluid (GCF) from each of the patients. One first preoperative sample was collected immediately before local anesthesia (A). The samples were taken from the periodontal pocket in cases where this partially perforated the retromolar trigone mucosa, and from the crevicular sulcus of the distal face of the second definitive molar in cases where the third molar was not visible. The other samples were collected immediately after surgery (B), one hour after surgery (C), 24 hours after surgery (D) and seven days after surgery when removing the sutures (E), all from the same location as the preoperative sample.
Samples were collected as follows: vacuum drying, isolation of the area with cotton rolls, mild air drying of the lower second or third molar, and placing the periopaper in the crevicular sulcus to obtain the sample of crevicular fluid or blood. This paper point has to be maintained in the above mentioned position for 30 seconds for crevicular fluid collection and 15 seconds for blood collection. Samples are then placed between the Periotron sensors to measure the amount of crevicular fluid obtained. The Periotron was previously calibrated to obtain microliters. Samples were put into a vial with a filter and this vial was preserved in dry ice until freezing at –80ºC.
In the laboratory, samples were treated using the ELISA assay, which is a "sandwich" enzyme immunoassay. The samples and the standards are incubated in microtiter wells, covered with a monoclonal anti-IL-6 antibody and in the presence of a second monoclonal anti-IL-6 antibody together with acetylcholinesterase. After incubation, the wells are washed and enzyme activity is measured with a chromogenic substrate. The intensity of the colour is proportional to the concentration of IL-6 in the sample or the standard.
In order to completely separate the sample and the Periopaper®, GCF was diluted from the paper points using filtration and centrifugation with buffer aliquots (50 mM phosphate buffer, pH 7.2). To summarize, 200 µl of the previously mentioned buffer were applied to each paper point and the tube was centrifuged at 8000 rpm for 10 minutes.
Reagents were prepared in the following manner:
The lyophilized IL-6 conjugate was reconstituted with the amount of distilled water as stated on the label of the vial, that is, 12 ml.
Diluent 2 was reconstituted with 6 ml of distilled water.
The wash solution was diluted with 950 ml of distilled water.
The lyophilized IL-6 standard was reconstituted with 0.5 ml of distilled water, obtaining 10 ng/ml.
The control serum was reconstituted with 1 ml of distilled water.
A standard curve was prepared before starting each assay. Once the standard curve was obtained, the assay carried out with the samples of gingival crevicular fluid in accordance with the established assay protocol. The results of the samples were calculated by interpolation of the standard curve prepared in the same assay as the samples. The results were expressed in log 10.
The statistical evaluation was made at the Data Processing Center of the Universidad Complutense de Madrid. The BMDP program was used to produce a detailed description of data, frequencies tables, bivariate graphics, t-Tests and repeated measures analysis of variance. For quantitative or continuous variables, Students t-Test (parametric test for comparing means) and the Mann-Whitney test (non-parametric test) were used. Chi-square test and Yates correction were used for qualitative or categorical variables.
Results
The samples were homogeneous for age, sex, side, position of the third molar and length of operation.
The mean age in the methylprednisolone group was 23.4 years and in the diclofenac group was 23.6, with no significant difference between them (Students t = 0.866; Mann-Whitney = 0.665).
With respect to sex, in the methylprednisolone group 73% were female against 27% male, and in the diclofenac group 52.8% were women and 47.2% men. The Chi-square test (P=0.07) and the Yachts correction (P=0.122) were used to determine the randomness of the sample, the variables being either qualitative or categorical, and demonstrating that the differences were not significant.
Regarding the side of surgery, in the methylprednisolone group this was the right-hand side in 56.8% of cases and the left-hand side in 43.2%. In the diclofenac group this was evenly distributed at 50% each, that is to say, there were no significant differences in the side operated on: Pearsons chi-square P=0.562 and Yachts correction P=0.730.
Regarding the position of the third molar, four positions were considered in relation to the axial axis of the second molar: Distal, vertical, mesial and horizontal. There were no significant differences in position (P=0.633), consequently this did not influence the results.
Lastly, the duration of the operation was measured in both groups in minutes from the beginning of the incision until the completion of the suture. The mean time in the methylprednisolone group was 11.4 minutes and the diclofenac group 10.9 minutes, the Student t-Test was 0.709 and the Mann-Whitney test 0.499; thus, this difference was not significant either.
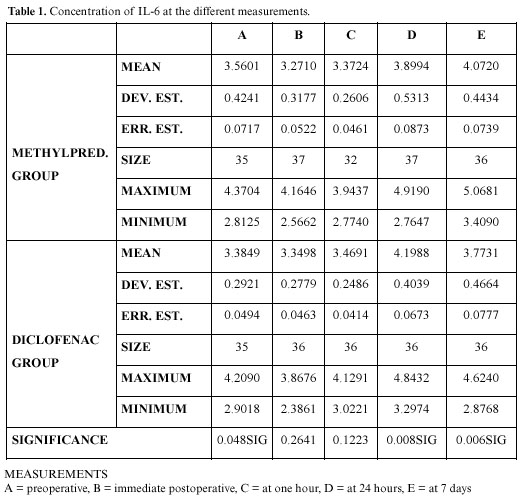
The behavior of the cytokine throughout the study was analyzed in both groups by descriptive statistics. Although we studied both the amount and the concentration of IL-6, for brevity we use the term concentration of IL-6, which is the amount of IL-6 divided by the total amount of gingival crevicular fluid. In table 1 we can see the behavior at each moment. The difference was significant 24 hours after surgery, when the concentration was higher in the diclofenac group (p<0.01), and 7 days after surgery, when a higher point was reached in the methylprednisolone group (p<0.01).
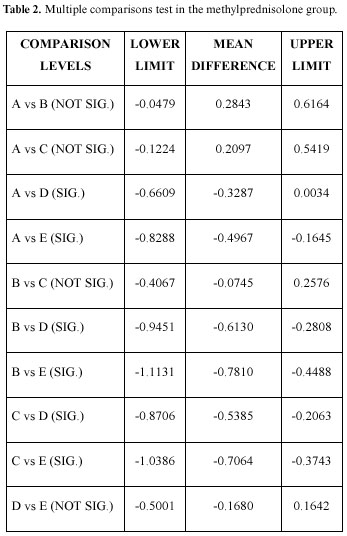
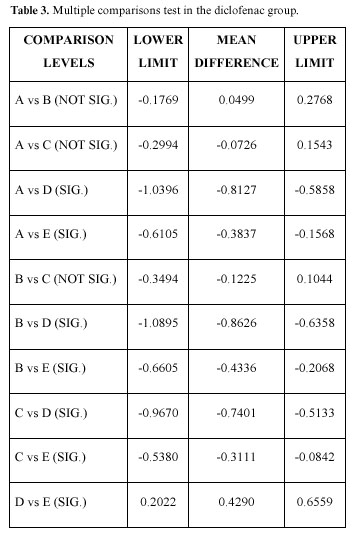
Table 2 shows the multiple comparisons test in the methylprednisolone group, in this case there were significant differences between the preoperative concentrations and those at 24 hours and 7 days; the preoperative, operative, and immediate postoperative concentrations being very similar. Likewise, the immediate postoperative, the 24 hour and 7 day differences are significant, as well as between those at one hour after the intervention and those at 24 hours and 7 days; there being no significant difference between the 24 hour and the 7 day measurements. Table 3 shows the diclofenac group (the only difference with the group that took glucocorticoids is that there was a significant difference between the 24 hour and the 7 day concentrations).
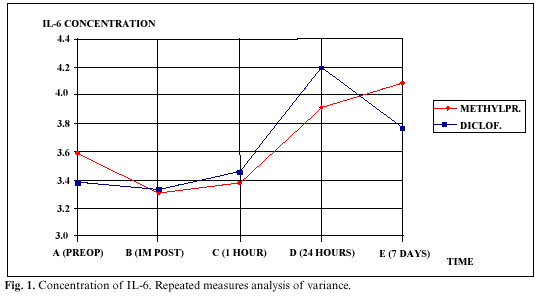
In figure 1, we can see the results obtained by the repeated measures analysis of variance. There were no significant differences between groups (p=0.530), but there were in the time effect (p=0) and the interaction effect (p=0.001), observing in figure 1 that the two lines are not at all parallel, in fact crossing on occasions.
Discussion
In the present study we have attempted to determine the presence of IL-6 after surgical extraction of lower third molars and the influence of the antiinflammatory used on the release of this cytokine.
In relation to the results obtained in both groups, both for clinical and laboratory variables, it is important to highlight that all samples were homogeneous in age, sex, side of surgery, location and position of the lower third molar, duration of surgery, and preoperative amount of IL-6 (although not concentration), so that any differences in postoperative results cannot be the result of the characteristics of the patient before surgery, nor to the surgical process itself, which was done in a regulated manner and always by the same surgeon. In fact, as inferred from Hollands studies (6), technical variations diminish if the surgery is always done by the same surgeon. The only significant difference during the preoperative period was that patients in the corticoid group showed a higher concentration of IL-6 than patients in the NSAID group. This difference was resolved with the repeated measures analysis of variance.
The amount and concentration of IL-6 was analyzed and significant differences were found 24 hours after surgery, when patients in the diclofenac group showed a higher amount and concentration of IL-6 (p<0.01), and 7 days after surgery, when this higher amount and concentration of IL-6 was found in the methylprednisolone group (p<0.01).
We are not certain why there is less IL-6 in the methylprednisolone group 24 hours after surgery. It may be due to the fact that corticoids inhibit IL-6 more than NSAID.
With the repeated measures analysis of variance we found that there were no significant differences in the amount and the concentration of IL-6 between groups. However, the effect over time was highly significant, that is, the differences between the five sample collection times when considering both groups together. The interaction effect was also significant, that is, each group behaved differently over time.
Several authors have found a correlation between the increase in IL-6 and postoperative complications, such as infections (7,8). The surgical trauma alone is enough for IL-6 release, while endotoxemia could be an additional stimulus for IL-6 concentration (9). We cannot corroborate the correlation between IL-6 and infection because there was no case of postoperative superinfection in our study.
According to Tonnesen (10), the routine measurement of IL-6 acts as a parameter for the identification of patients who may develop postoperative complications and will therefore require more careful postoperative monitoring. In other words, the association of a large increase in postoperative levels of IL-6 in plasma with postoperative complications may have prognostic significance (11).
In these patients with complications, the concentration of IL-6 is not only higher, but is also present for longer. The detection of an extended presence of IL-6 indicates that this is actively released for several days, because it has a short half-life. Di Padova(12) detected IL-6 one hour after starting the operation (cholecystectomy), remaining significantly high 72 hours later. We have found a high level of IL-6 even 7 days after surgery, when there is still some inflammation in the body.
While we have detected IL-6 after surgical extraction of lower third molars, other authors have done so in burn lesions or after elective surgery (13, 14). Given that IL-6 is a mediator of the acute stage reaction in human beings (4), it is reasonable to believe that an increase in IL-6 levels in serum after surgery is related to the degree of tissue damage. This has been demonstrated in several studies on operated patients, IL-6 being an early and sensitive marker of surgical damage. In general, the greater the surgical damage, the stronger the IL-6 response in serum and the higher the concentration (15).
The high increase of IL-6 24 hours after lower third molar surgery indicates that this procedure involves significant surgical trauma.
Since this cytokine is one of the most important ones involved in the physiological response to trauma, inflammation and infection, a great investigative effort is being made in order to find methods to regulate the systemic effects of IL-6 and improve postoperative control. Glucocorticoids have already been tested in experimental and clinical studies. The endogenous and exogenous increase in the levels of glucocorticoids in blood, diminishes the production of IL-6 in human and animal studies (16).
Several researchers have obtained satisfactory results using glucocorticoids and NSAID for the inhibition of IL-6. Teoh (17), for example, detected a lower concentration of IL-6 in patients who received 250 mg i.v. of methylprednisolone after heart bypass surgery. Also in bypass surgery, Engelman (18) observed a decrease in IL-6 in patients treated with one gram of methylprednisolone before surgery and 4 mg of dexamethasone every 6 hours after, these patients leaving the ICU one day earlier than usual. Osaka (19) reached the same conclusions in neurosurgery, having lower levels of IL-6 and a better postoperative in patients treated with glucocorticoids.
We must bear in mind the difference between the doses of corticoids administered in the previous examples and those administered in our study (only 12 mg/day), which explains why the results of our study are not as striking as in the above examples.
In addition to trying to regulate the production of IL-6 with corticoids, other preparations with fewer side-effects are being studied, such as ibuprofen and other NSAID (20).
We have only made reference to general surgery because very few studies exist on the relationship of IL-6 with Oral Surgery. We have found no study on IL-6 and surgery of the lower third molar. IL-6 has been related with the pathogenesis of temporomandibular disorders (inducing osseous changes in the condyle) (21,22).
Only one group of researchers has found a relationship between IL-6 and oral and maxillofacial surgery (but not specifically with lower third molar surgery). These are Miyawaki et al. (3, 23) who found a relationship between IL-6 and cystectomy, benign tumor extirpation, radical surgery for cancer of the tongue, palate, mandible and floor of the mouth, as well as bone grafts. They also found a relationship between the increase in IL-6 and the presence of postoperative fever, as well as with the duration of surgery (although the average duration of these radical interventions was 9 hours). These authors state that contamination by oral bacteria has an influence on IL-6 release when comparing maxillofacial surgery with abdominal surgery,.
After seeing so many and varied studies, we can conclude that much research remains to be carried out in order to determine whether IL-6 concentration in serum simply reflects the level of tissue damage or plays a more active role in the host defense mechanism or in the induction of postoperative complications.
Conclusions
1.- After surgical extraction of lower third molars there was a significant increase in IL-6 levels in both groups, which was evident 24 hours after surgery, remaining high after 7 days.
2.- Patients treated with NSAID showed a higher amount of IL-6 during the first day with a decrease at the following control.
3.- In contrast to the above results, patients treated with methylprednisolone showed the highest levels of IL-6 after 7 days.
Acknowledgments: This study was carried out with a grant for investigation from the Autonomous Community of Madrid (Spain).
References
1. Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of IL-6 in gingival crevicular fluid from adults with destructive periodontal disease. J Periodontol 1994;64:980-3. [ Links ]
2. Soskolne WA, Sela MN, Offenbacher S, Barak V. Secretion of PGE 2, IL-1ß, IL-6, TNFa by adherent mononuclear cells from early onset periodontitis. J Periodontol 1994;65:139-46. [ Links ]
3. Miyawaki T, Maeda S, Shimada M. Elevation of plasma interleukin-6 level in patients undergoing oral and maxillofacial surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:15-20. [ Links ]
4. Cruickshank AM, Fraser WD, Burns JG. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci 1990;79:161-5. [ Links ]
5. Claman H, Tilles SA. Immunosuppressive and antiinflammatory actions of corticosteroids. En: Austen, K.F. editor. Therapeutic immunology. Cambridge: Blackwell Science; 1996. p. 105-18. [ Links ]
6. Holland CS. The influence of methylprednisolone on post-operative swelling following oral surgery. Br J Oral Maxilofac Surg 1987;25:293-9. [ Links ]
7. Baigrie RJ, Lamont PM, Whiting S, Morris PJ. Portal endotoxin and cytokine responses during abdominal aortic surgery. Am J Surg 1993;166:248-51. [ Links ]
8. Saklatvala J. Interleukin-1 and connective tissue. En: Dingle, J.T. editor. Interleukin-1, inflammation and disease. Amsterdam: Elsevier; 1989. p. 143-62. [ Links ]
9. Wortel CH, van Deventer SJH, Aarden LA. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery 1993;114:564-70. [ Links ]
10. Tonnesen E, Christensen VB, Toft P . The role of cytokines in cardiac surgery. Int J Cardiol 1996;53 suppl.:1-10. [ Links ]
11. Tsang TM, Tam PKM. Cytokine response of neonates to surgery. J Pediat Surg 1994; 29:794-7. [ Links ]
12. Di Padova F, Pozzi C, Tondre MJ, Tritapepe R. Selective and early increase of IL-1 inhibitors, IL-6 and cortisol after selective surgery. Clin Exp Immunol 1991;85:137-42. [ Links ]
13. McBride WT, Armstrong MA, McBride SJ. Immunomodulation: an important concept in modern anaesthesia. Anesthesia 1996;51:465-73. [ Links ]
14. Struzyna J, Pojda Z, Braun B, Chomicka M, Sobiczewska E, Wrembel J. Serum cytokine levels (IL-4, IL-6, IL-8, G-CSF. GM-CSF) in burned patients. Burns 1995;21:437-40. [ Links ]
15. Bellón JM, Manzano L, Bernardos L. Cytokine levels after open and laparoscopic cholecystectomy. Eur Surg Res 1997;29:27-34. [ Links ]
16. Tonnesen E, Wanscher M, Hóhndorf K. et al. Effect of methylprednisolone on the cytokine response in patients undergoing lung surgery. Acta Anaesthesiol Scand 1993;37:410-4. [ Links ]
17. Teoh KH, Bradley CA, Gauldie J et al. Steroids inhibition of cytokine mediates vasodilation after warm heart surgery. Circulation 1995;92:347-53. [ Links ]
18. Engelman RM, Rouson JA, Flack JE et al. Influence of steroids on complement and cytokine generation after cardiopulmonary bypass. Ann Thorac Surgery 1995;60:801-4. [ Links ]
19. Osuka K, Suzuki Y, Saito K. Changes in serum cytokine concentrations after neurosurgical procedures. Acta Neurochir 1996;138:970-6. [ Links ]
20. Chambier C, Chassard D, Bienvenu J. Cytokine and hormonal changes after cholecystectomy. Ann Surg 1996;224:178-82. [ Links ]
21. Kaneyama K, Segami N, Sato J, Nishimura M, Yoshimura H. Interleukin-6 family of cytokines as biochemical markers of osseous changes in the temporomandibular joint disorders. Br J Oral Maxillofac Surg 2004;42:246-50. [ Links ]
22. Nishimura M, Segami N, Kaneyama K, Sato J, Fujimura K. Comparison of cytokine level in synovial fluid between successful and unsuccessful cases in arthrocentesis of the temporomandibular joint. J Oral Maxillofac Surg 2004;62:284-7. [ Links ]
23. Miyawaki T, Maeda S, Koyama Y, Fukuoka R, Shimada M. Elevation of plasma interleukin-6 level is involved in postoperative fever following major oral and maxillofaccial surgery. Oral Surg Oral Med Oral Path Oral Radiol Endod 1998;85:146-52. [ Links ]
![]() Correspondence:
Correspondence:
Dra. Carmen López Carriches
C/. Rey Francisco, 11. Bajo izda, 28008 Madrid
E-mail: carmen.lopez@uem.es
Received: 14-08-2005
Accepted: 7-07-2006