INTRODUCTION
In December 2019, an outbreak of pneumonia was detected in the Chinese city of Wuhan caused by a novel coronavirus. It was named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) due to its genetic link to the coronavirus that caused the SARS outbreak in 20031. The World Health Organisation (WHO) named the disease caused by this virus COVID-19, which stands for Coronavirus Disease 2019. In just a few months, the virus had spread around the globe. It was declared a pandemic on 11 March 20202.
Most COVID-19 patients are asymptomatic or present with mild symptoms such as a cough, fever, fatigue or myalgias, mainly only requiring symptomatic treatment in the form of analgesics and antipyretics. However, between 10-15% of infected patients present with severe respiratory symptoms, developing hypoxemic respiratory failure requiring hospitalisation for mechanical ventilation. Between 3 and 5% of infected patients will need to be admitted to an Intensive Care Unit (ICU)3.
With the information available to date, experts have identified two phases in the disease’s development. In the first phase, there is a specific response by the immune system which aims to eliminate the virus and stop its reproduction. However, if the body is unable to halt this process, a serious inflammatory response takes place, triggering a massive release of cytokines4. The increase in inflammatory markers induces pulmonary fibrosis, breathing difficulties, reduced oxygen saturation (SatO2) and systemic lesions, leading to acute respiratory distress syndrome (ARDS)5. Higher levels of inflammatory cytokines have been linked to increased severity and poorer prognosis6, with ARDS being the main cause of death in patients suffering from COVID-197.
Due to the hyperinflammation that takes place during the second phase of this disease, various studies have shown that corticosteroids may be useful in the treatment of this pathology5,8,9.
The results of the RECOVERY randomised clinical trial, which studied dexamethasone treatment, were recently published. In this trial, more than 2000 patients were treated with 6 mg of dexamethasone once a day, and this group was compared to more than 4000 patients receiving standard treatment. The preliminary results showed that dexamethasone reduces the mortality rate by one-third in patients requiring mechanical ventilation, and by one-fifth in patients requiring oxygen alone10.
Based on this new evidence, dexamethasone was the corticosteroid treatment strategy employed in the centre where this study was carried out, beginning with doses of 6 mg/day intravenously (IV) or orally (PO) over the course of 10 days. In the event of clinical worsening, dexamethasone treatment was increased to 8 mg every 8 hours, administered intravenously.
During the study period, owing to the centre's experience in the use of corticosteroids during the first months of the pandemic, a strategy change took place in the hospital's protocol as it was not obtaining the expected results. The new treatment strategy involved initiating treatment with high doses of corticosteroids before decreasing to lower doses at the discretion of the medical professional. In this case, methylprednisolone was considered as the first treatment option as it was the corticosteroid used during the first stage of the pandemic, producing very good results, starting treatment with a regimen of 125-250 mg/day intravenously over the course of 3 days5. This was then reduced to low doses of methylprednisolone, with a regimen of 40 mg every 12 hours. Patients admitted to ICUs with mild symptoms were administered low doses of methylprednisolone directly.
COVID-19 remains a relatively unknown disease, owing to its recent appearance and the fact that its treatment is continuously evolving. It is essential, therefore, to continue researching the disease’s behaviour and the possible pharmacotherapeutic options.
The aim of this study was to analyse the impact of the change in strategy from a low-dose to a high-dose corticosteroid on the ICU admission rate of patients hospitalised due to COVID-19.
MATERIAL AND METHODS
An observational, retrospective study was designed to evaluate the effect on the ICU admission rate of the change in corticosteroid treatment strategy set out in the treatment protocol for COVID-19 patients admitted to a tertiary hospital. The study was carried out between 17 August 2020 and 1 November 2020, enrolling all patients of legal age who had been diagnosed with moderate-to-severe COVID-19 who required hospital admission and for whom corticosteroid treatment was indicated.
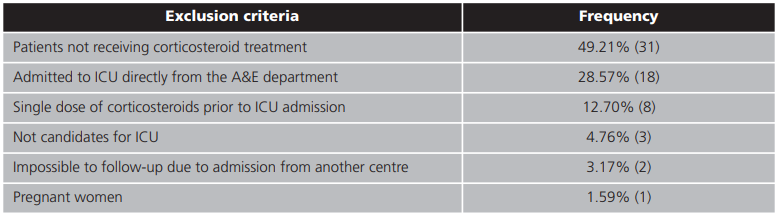
Exclusion criteria included patients who did not require corticosteroid treatment, patients who were admitted directly to the ICU from an A&E department, and patients who had received a single dose of corticosteroid prior to ICU admission. Patients who could not be followed up due to admission from another hospital, non-intensive patients and pregnant women were also excluded from the study.
The diagnosis of COVID-19 was confirmed with the identification of SARS-CoV-2 in at least one sample of nasopharyngeal exudate by means of the RT-PCR (reverse transcription polymerase chain reaction) analytical technique.
The pharmacotherapeutic management of patients diagnosed with COVID-19 was carried out in accordance with the hospital's own protocol, previously agreed upon by the Internal Medicine (Infectious Diseases Unit), Intensive Medicine, Hospital Pharmacy, Pneumology and Paediatrics departments. In this protocol, the hospital admission criteria indicative of moderate-to-severe disease were: the presence of respiratory symptoms and dyspnoea, SatO2 <94% or patients with mild symptoms categorised as high-risk [aged ≥60 years or suffering from comorbidities (diabetes mellitus (DM), hypertension, COPD, cardiovascular disease, hepatopathies, neoplasia, immunosuppression, etc.)] for whom it was not possible to actively follow-up via telephone on a daily basis. Similarly, corticosteroid treatment indication criteria were defined as follows: patients with severe symptoms who required mechanical ventilation, ECMO (extracorporeal membrane oxygenation) or oxygen therapy, particularly if more than seven days have passed since the onset of symptoms.
The clinical and demographic data of all patients enrolled in the study were collected by consulting their computerised medical history via the Orion Clinic® program. All patients were followed-up from hospital admission through to discharge or death, The data collected were: demographic data [age, gender and comorbidities (DM, hypertension, obesity and dyslipidaemia) and analytical data [C-reactive protein (CRP), fibrinogen, D-dimers, lactate dehydrogenase (LDH), ferritin and interlukin-6 (IL-6)] prior to corticosteroid treatment. Data were also were collected on the length of hospital stay, the need for ICU admission and the duration of admission to the ICU, as well as all the drugs taken for the treatment of the disease included in the protocol, both corticosteroid therapy (dexamethasone or methylprednisolone) and thromboembolic prophylaxis, the use of antibiotics and the administration of tocilizumab, anakinra and remdesivir.
The main variable in the study was the ICU admission rate following the change in corticosteroid treatment strategy made in the centre's protocol.
Two study arms were established, comparing patients treated initially with low doses of corticosteroids (dexamethasone 6 mg/24 h) with patients who received high doses (methylprednisolone 125-250 mg/24 h) at the beginning of corticosteroid treatment.
The SPSS Statistics® software was used for the statistical analysis, always considering a p-value of <0.05 as the threshold of statistical significance. Quantitative variables were summarised using the mean (95% confidence interval) or median (interquartile range, IQR) values as appropriate for data distribution, while categorical variables were summarised using absolute and relative frequencies. To study the correlation between the type of corticosteroid treatment employed and ICU admission rate, a binary logistic regression model was constructed which included the following variables that could lead to confusion or affect the response: gender, age, comorbidities (DM, hypertension, dyslipaemia) and analytical data (CRP, fibrinogen, D-dimer, LDH, ferritin and IL-6). In order to be introduced in the model, the quantitative variables were categorised based on the best cut-off point obtained for ICU admission from the corresponding ROC curve.
RESULTS
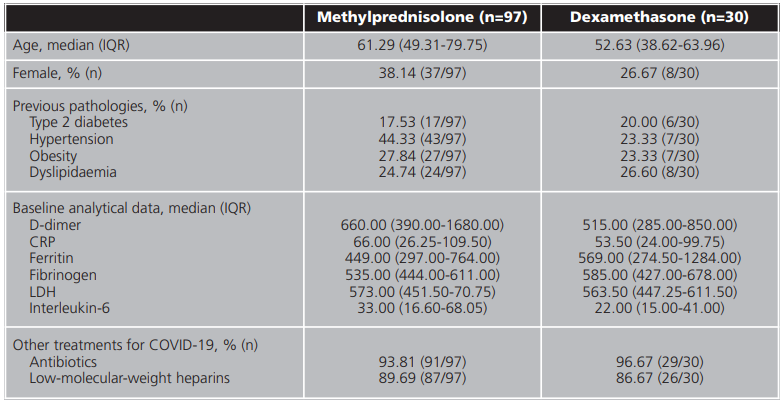
During the study period (15 August 2020 - 1 November 2020), 190 patients were admitted for COVID-19. Of these, a total number of 127 patients (66.84%) were enrolled in this study. The exclusion criteria for the remaining patients are set out in table 1. The baseline characteristics of the patients enrolled in the study are set out in table 2. Patients' median age was 61.29 years (IQR = 49.31-79.75 years). Of all patients, 7.9% (n=10) died during the study period.
Table 2. Baseline characteristics of patients enrolled in the study based on the corticosteroid administered
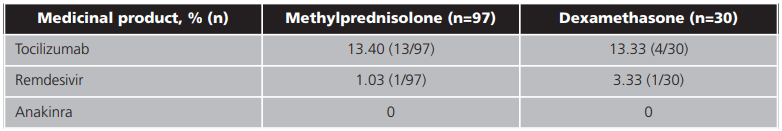
Tocilizumab was administered to a small number of patients (table 3) in both study arms after clinical worsening and lack of response to corticosteroid therapy. Anakinra, another of the biological treatment options included in the centre's protocol, was not administered to any of the patients enrolled during the study period, although it was considered.
In both groups, patients received a minimum of two doses of corticosteroids during admission.
12.4% (12/97) of patients administered methylprednisolone were later admitted to an ICU, compared to 30.0% (9/30) of those administered dexamethasone.
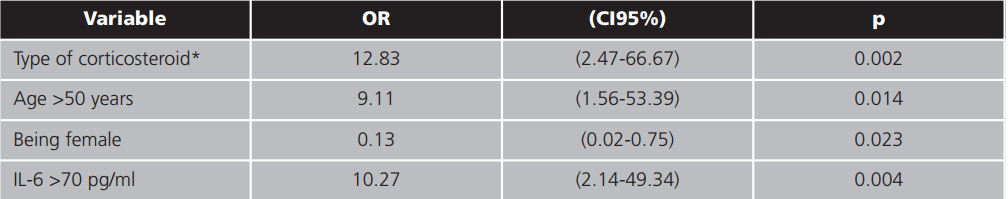
In the constructed logistic regression model, corticosteroid therapy (administration of dexamethasone), gender, being older than 50 and an IL-6 level >70 pg/ml remained predictors of ICU admission. The results of this model are set out in table 4.
DISCUSSION
The RECOVERY trial compared the administration of 6 mg/day of dexamethasone over the course of 10 days with the standard treatment in 6,425 patients who had been admitted to hospital for SARS-CoV-2 infection. The survival rate was significantly higher in patients who were treated with dexamethasone, particularly in the sub-arm of 1,007 patients who received invasive mechanical ventilation10. Based on the findings of the RECOVERY trial, two additional corticosteroid trials focusing on ICU patients were prematurely stopped after enrolment11,12.
Current evidence on the pharmacological principles that guide the administration of corticosteroids for the treatment of ARDS is scarce. This lack of information may have led to the heterogeneity of treatment protocols and a misinterpretation of the available results.
Corticosteroids are agonist compounds that bind to the glucocorticoid receptor (GR) to produce their pharmacological response. The clinical efficacy depends on the magnitude and duration of exposure to the GR.
Corticosteroid therapy seeks to support the central regulatory function of the activated glucocorticoid receptor α (GC-GRα) during the development and resolution of the disease. The deregulated immune response observed in the case of COVID-19 is qualitatively similar to that of multifactorial ARDS13.
In patients with severe COVID-19, GR expression in bronchoalveolar lavage myeloid cells has a negative correlation with neutrophilic inflammation of the lung and the severity of symptoms14.
Corticosteroids cross the bisphospholipid cell membrane due to their lipophilic composition, binding to the GR in the cytoplasm. Once activated, two pathways are identified in the mechanism of action: genomic and non-genomic.
In the genomic mechanism, GR undergoes a conformational change. It is activated and transferred to the nucleus, where it activates or deactivates gene transcription by means of corticosteroid response elements.
Some of the anti-inflammatory effects are caused by this genomic mechanism of "transrepression", where GR coupled with its ligand interferes with the activation of transcription factors, the production of pro-inflammatory cytokines and factors for white blood cell maturation. We speak of “transactivation” to refer to the increased synthesis of anti-inflammatory molecules. It is important to note that this mechanism can take hours or even days to manifest15,16.
Genomic effects usually predominate at low doses of corticosteroids.
Non-genomic mechanisms are immediate (taking seconds or minutes) and are caused by physical-chemical interactions. These are due to the activation of intracellular signalling cascades mediated by kinases with an anti-inflammatory effect. Rapid non-genomic actions include anti-inflammatory regulation e.g., inhibition of neutrophil degranulation and production of macrophage superoxide anions and vasomotor regulation (such as enhanced systemic response to norepinephrine and local induction of endothelial nitric oxide).
Non-genomic effects are generally triggered at substantially higher doses than genomic effects, intensifying in line with the increasing dose until they stabilise with high-dose therapy17.
In the early stages of the pandemic, high-dose corticosteroid pulse therapies were widely used in Spain to treat COVID-19, but not in other countries, hence the lack of published evidence.
These therapies are employed to halt the process of systemic18 inflammation that develops in certain patients suffering from severe COVID-19. Some studies5,19,20have already described the positive effects of corticosteroid pulse therapies on mortality rate in patients suffering from severe COVID-19.
ICU admission rate was chosen as a variable because the space in intensive care units is limited, and because overcrowding of ICUs would result in poorer healthcare and the possibility of a higher mortality rate.
The percentage of hospitalised COVID patients requiring ICU admission varies according to published studies. These range from 23-35% according to the US government's Center for Disease Control and Prevention to 9.3% according to the Spanish Society of Internal Medicine's SEMI-COVID-1921 Registry, 16% in Italy22 and 17% according to a British cohort23.
Given the results obtained for ICU admission rate with the low-dose corticosteroid strategy (30% of hospitalised patients), it was postulated that the effect of the high-dose corticosteroid strategy could be better than that of the low-dose strategy since corticosteroids act on different pathways in the former (genomic vs. non-genomic), combining both effects.
In this study, a significant improvement was noted in the ICU admission rate of patients treated with the high-dose corticosteroid strategy (methylprednisolone) compared to patients treated with the low-dose corticosteroid strategy (dexamethasone).
Besides corticosteroid therapy (administration of dexamethasone), other predictors of ICU admission such as being male, being over 50 years old and having an IL-6 level >70 pg/ml were detected in the logistic regression model constructed, as was expected.
Some of the main limitations of this study are its retrospective, observational design, the low number of enrolled patients and the fact that the main variable is a process variable (ICU admission), which makes it difficult to draw solid conclusions about the benefit of using high doses of corticosteroids (methylprednisolone) as opposed to low doses (dexamethasone).
CONCLUSIONS
The high-dose corticosteroid strategy is better than the low-dose corticosteroid strategy for the treatment of severe COVID-19, given the biological bases shown and the results obtained in this study, in which the ICU admission rate was lower. This hypothesis must be confirmed in randomised, prospective studies with a greater number of patients.