Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 no.1 Redondela ene./mar. 2009
| Review |
Role of the pharmacist in pre-exposure chemoprophylaxis (PrEP) therapy for HIV prevention
Papel del farmacéutico en la quimioprofilaxis pre-exposición para prevención del VIH
Kevin A. CLAUSON, Hyla H. POLEN, Shine A. JOSEPH, Antonia ZAPANTIS.
| ABSTRACT With a global estimate of 2.5 million new infections of HIV occurring yearly, discovering novel methods to help stem the spread of the virus is critical. The use of antiretroviral chemoprophylaxis for preventing HIV after accidental or occupational exposure and in maternal to fetal transmission has become a widely accepted method to combat HIV. Based on this success, pre-exposure chemoprophylaxis (PrEP) is being explored in at-risk patient populations such as injecting drug users, female sex workers and men who have sex with men. This off-label and unmonitored use has created a need for education and intervention by pharmacists and other healthcare professionals. Pharmacists should educate themselves on PrEP and be prepared to counsel patients about their means of obtaining it (e.g. borrowing or sharing medications and ordering from disreputable Internet pharmacies). They should also be proactive about medication therapy management in these patients due to clinically important drug interactions with PrEP medications. Only one trial exploring the safety and efficacy of tenofovir as PrEP has been completed thus far. However, five ongoing trials are in various stages and two additional studies are scheduled for the near future. Unfortunately, studies in this arena have met with many challenges that have threatened to derail progress. Ethical controversy surrounding post-trial care of participants who seroconvert during studies, as well as concerns over emerging viral resistance and logistical site problems, have already halted several PrEP trials. Information about these early trials has already filtered down to affected individuals who are experimenting with this unproven therapy as an evening before pill. The potential for PrEP is promising; however, more extensive trials are necessary to establish its safety and efficacy. Pharmacists are well-positioned to play a key role in helping patients make choices about PrEP, managing their therapy, and developing policy with an eye towards the future. Key words: Acquired Immunodeficiency Syndrome. Chemoprevention. United States. | RESUMEN Con una estimación global de 2,5 millones de nuevas infecciones de VIH cada año, es crítico descubrir nuevos métodos para ayudar a detener la extensión del virus. El uso de quimioprofilaxis antirretroviral para prevención del VIH después de una exposición accidental u ocupacional y en transmisión materno-fetal se ha convertido en un método comúnmente aceptado para combatir el VIH. Basando en este éxito, se está explorando la quimioprofilaxis pre-exposición (PrEP) en poblaciones de riesgo tales como drogadictos intravenosos, trabajadoras sexuales, y hombres que tiene sexo con hombres. Este uso no autorizado y no vigilado ha creado la necesidad de educación e intervención de farmacéuticos y otros profesionales de la salud. Los farmacéuticos deberían formarse sobre la PrEP y estar preparados para aconsejar a los pacientes sobre los medios de obtenerla (p.e. prestándose o compartiendo medicaciones y comprándola en farmacias de confianza en Internet). También deberían ser proactivos en el seguimiento de estos pacientes debido a las importantes interacciones de los medicamentos PrEP. Solo se ha completado un ensayo que exploró la seguridad y la eficacia del tenofovir como PrEP. Sin embargo, están programados 5 ensayos en marcha en diversos estadios, y dos estudios adicionales en un futuro próximo. Desafortunadamente, los estudios en este campo se han encontrado con muchos obstáculos que han amenazado interrumpir su progreso. La controversia ética sobre la atención post-ensayo de los pacientes que se seroconvierten durante el estudio, así como las preocupaciones sobre la emergente resistencia viral y los problemas logísticos, ya han interrumpido varios ensayos de PrEP. La información de estos primeros ensayos se ha filtrado e los individuos afectados que están experimentando con este tratamiento no probado como una píldora de la tarde anterior. El potencial de la PrEP es prometedor, sin embargo se necesitan ensayos más extensos para establecer su seguridad y eficacia. Los farmacéuticos están bien posicionaos para jugar un papel importante ayudando a los pacientes a tomar mejores decisiones sobre la PrEP, gestionando su tratamiento, y desarrollando políticas con un ojo en el futuro. Palabras clave: Síndrome de inmunodeficiencia adquirida. Quimioprofilaxis. Estados Unidos. |
Kevin A. CLAUSON. Pharm.D. Associate Professor. College of Pharmacy – West Palm Beach, Nova Southeastern University. Palm Beach Gardens, FL (United States).
Hyla H. POLEN. Pharm.D. Lead Consultant, Integrated Consulting Associates. Jupiter, FL (United States).
Shine A. JOSEPH. MBA, Pharm.D. Specialty Resident in Drug Information. College of Pharmacy – West Palm Beach, Nova Southeastern University. Palm Beach Gardens, FL (United States).
Antonia ZAPANTIS. Pharm.D. Assistant Professor. College of Pharmacy, Nova Southeastern University. Fort Lauderdale, FL (United States).
Received (first version): 22-Sep-2008
Accepted: 21-Nov-2008
BACKGROUND
With a global estimate of 2.5 million new infections of HIV occurring yearly and even more undiagnosed cases waiting to be discovered, research into methods of HIV transmission prevention is a critical step in combating the global AIDS pandemic.1 Pre-exposure chemoprophylaxis (PrEP) is being explored as a novel strategy to stem the spread of HIV in at-risk patient populations such as men who have sex with men (MSM), injecting drug users (IDU), and female sex workers. While the timing differs, the end goal of preventing HIV transmission via PrEP is similar to that of post-exposure prophylaxis (PEP). PEP is the practice of taking antiretrovirals (ARVs) to prevent infection after a potential or inadvertent exposure (e.g. accidental occupational needle-stick or victim of sexual assault). PEP has been a widely accepted practice by healthcare practitioners for many years.2,3 As the name implies, PrEP simply transitions the concept of chemoprophylaxis use after an exposure event, and instead, attempts to prevent transmission before the exposure occurs. The impetus for the development of PrEP was the extensive and increasingly successful use of prophylaxis for mother-to-infant transmission with ARVs as well as promising animal studies that repeatedly exposed primates receiving antiretroviral PrEP to an HIV-like virus without actual disease transmission.4
PrEP is not a universally agreed upon approach for preventing the spread of HIV as its acceptance has been marred by controversy and it has even been labeled as the evening before pill.5 More alarmingly, PrEP trials have consistently prompted public protest demonstrations centered around the ethical appropriateness of the studies, forcing the halt of several trials in Asia and Africa.6,7 In addition to ethical considerations, at least one trial has been prematurely discontinued due to concerns that PrEP use could complicate future treatment of HIV-infected individuals.8 However, there are reasons that PrEP is still being actively pursued as a potential weapon in our pharmacological arsenal against HIV. Two of the prime reasons that PrEP has generated interest include encouraging preclinical data and its potential for positive global impact.
CHEMOTHERAPEUTIC AGENTS USED IN PrEP
No medication has been approved by the Food and Drug Administration (FDA) for use as PrEP in the United States (US). Nor is PrEP listed as a non-FDA approved use in commonly used drug compendia.9-12 The earliest published study for PrEP was a Phase I/II safety and pharmacokinetic study using nevirapine. However, no efficacy data for nevirapine has been reported since the original publication and efforts have since shifted to tenofovir-based approaches.13-22 Specifically, tenofovir (also known as tenofovir disoproxil fumarate or TDF) alone and Combo-PrEP (as tenofovir and emtricitabine) have been studied, with TDF plus emtricitabine and efavirenz, garnering some interest as an agent for PrEP.23,24 Additionally, two newer agents, etravirine and maraviroc, also used to treat HIV, have demonstrated high enough concentrations in blood or tissue to raise interest in their use as PrEP.25-27 All oral agents studied were at normal FDA approved doses for treatment of HIV infection. Other potential compounds for PrEP and the criteria for selecting them have been detailed elsewhere.14
POPULATIONS STUDIED/SURVEYED
The increasing interest in PrEP as a method to curtail the continuing spread of the HIV epidemic is illustrated by the number of studies that have been initiated and planned within the last several years. Current, completed, and ongoing studies of PrEP are primarily being conducted in Sub-Saharan Africa, Southeast Asia and South America.15-17 A trial in the US is also being coordinated between centers in San Francisco, Boston and Atlanta.16 These studies are focusing on parameters including safety and efficacy and the effects of PrEP on HIV risk behaviors across populations, including IDU, MSM, female sex workers, serodiscordant couples (i.e. where one partner is HIV positive and the other is not), and heterosexual men and women.
Studies
Completed/Halted Studies
The only completed study on the efficacy and safety of PrEP was a double-blind, randomized, placebo-controlled trial conducted by Family Health International (FHI) and funded by the Bill & Melinda Gates Foundation (BMGF).15 The study participants were 936 HIV negative women between the ages of 18 and 35 at high risk for HIV infection in Ghana, Cameroon, and Nigeria. High-risk was defined as an average of three to four coital acts per week and four or more sexual partners per month. Patients were randomized to receive either oral 300 mg of TDF or oral placebo for up to a 12 month follow up between June 2004 and March 2006. Safety was assessed by monitoring for elevated blood levels of phosphorous, creatinine, and aspartate aminotransferase or alanine aminotransferase, and the efficacy endpoint was HIV-1 or HIV-2 infection. Unfortunately, the Cameroon and Nigeria sites were closed before the trial ended, which limited the power of the study. Because the trial was unable to enroll the targeted 400 participants per site, the number of evaluated person-years of follow up was insufficient to draw conclusions about efficacy. The efficacy in 859 patients was analyzed using 232.6 person-years of follow up in the TDF group and 241.3 person-years in the placebo group. Two seroconversions occurred in the TDF arm (rate=0.86 per 100 person-years) versus six seroconversions in the placebo arm (rate=2.48 per 100 person-years), yielding a total of eight newly diagnosed HIV infections among the participants, rate ratio of 0.35 (95%CI=0.03 – 1.93; p=0.24). There was no indication of resistance to TDF from the one seroconverted participants blood that was available for testing.
Safety was assessed using 210.2 person-years in the TDF group and 217.6 person-years in the placebo group, which revealed no significant differences between the TDF and placebo arms in clinical or laboratory safety outcomes, and the investigators were able to conclude that use of oral TDF in HIV negative women did not show an increase in the adverse events that were evaluated. The investigators found that sexual behavior changed during the study period. From the time of screening to trial follow up, the average number of coital acts per week rose from 12 to 15, but the number of sex partners in the previous 30 days fell from 21 to 14. Additionally, average condom use improved from 52% at the time of screening to 92% at follow up. Of note, TDF was discontinued during the 52 pregnancies per 100-person years that were reported during the trial. The investigators hypothesized that these changes were likely due to the intense counseling that the participants received during the trial on safer sex practices and the likelihood that the act of taking a daily pill resulted in an increased incidence of behavior modification in order to prevent HIV infection.
To date, four PrEP trials have been halted due to public protest, ethical and medical concerns, and site logistics.6,8 Two trials, one each in Cambodia and Cameroon, have been stopped due to outrage about the perception of unethical treatment of study participants6, and public protest consequently led to the closing of the studies by the government of each country. The Cambodian trial, funded by the National Institutes of Health (NIH) and BMGF, ended when concerns were raised about the level of healthcare being provided to the study participants. Issues in question included counseling services, insurance provision, and medical follow-up for participants who seroconverted during the trial. Soon to follow, the Cameroonian trial, led by the FHI, was ceased due to similar protests and allegations. Data from patients already participating in this study were included in the results of the Ghana trial.
Another trial that was halted before enrollment could take place was scheduled to commence in 2005 in Malawi.8 Government officials stopped it citing a fear that resistance would develop to TDF, rendering it useless as a future treatment option for HIV. Finally, a trial taking place in Nigeria was halted by the sponsor, FHI, at the recommendation of the independent Data and Safety Monitoring Committee overseeing the study due to insurmountable problems with the operational and laboratory facilities.6 This trial was discontinued after 100 patients had already been randomized into the program and data from those patients were also incorporated into the results for the Ghana study. An overview of all of the completed and halted trials discussed is available in Table 1.
Ongoing Studies
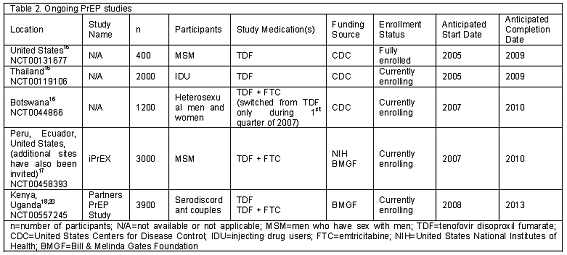
While the controversy surrounding PrEP has compromised many study efforts, there are still several ongoing international trials in various stages of planning and completion.16,17 One study currently underway in the US targets the MSM populations in Boston, San Francisco and Atlanta with an expected completion date of 2009.16 The Centers for Disease Control and Prevention (CDC) sponsored study is fully enrolled with 400 HIV negative, high-risk participants. Subjects were randomized to receive either TDF or placebo immediately or to receive either TDF or placebo after nine months. The study objectives are to determine the clinical and behavioral safety of PrEP, and the investigators will attempt to compare risk behaviors among the four arms.
An even more ambitious trial of PrEP in MSM is the iPrEx study, which has enrolled over 700 participants and is targeting 3000 participants total.17 This study, sponsored by several agencies, including the NIH and BMGF, has sites in Peru, Ecuador, and the US, with several other countries invited to participate. The study plans on evaluating TDF plus emtricitabine versus placebo and will endeavor to establish both safety and efficacy of PrEP in high-risk MSM.
Two additional CDC sponsored safety and efficacy studies are currently enrolling participants: one in Thailand and one in Botswana.16 The Thailand study is investigating TDF versus placebo in 2400 IDU being recruited at 17 centers throughout Bangkok. The Botswana trial is currently in the process of enrolling 1200 heterosexual men and women to investigate TDF plus emtricitabine. The Thailand study is scheduled to conclude in 2009 and the Botswana study will finish in 2010. And, finally, the Partners PrEP Study, funded by BMGF, is currently enrolling 3900 serodiscordant couples in Kenya and Uganda to study the safety and efficacy of TDF and TDF plus emtricitabine and is scheduled to conclude in 2013.18,20 Further details of all of the ongoing studies discussed are provided in Table 2.
Planned Studies
Additional studies are currently in the pipeline for PrEP focusing on two separate populations.18-21 The FEM-PrEP trial, to be conducted in Kenya, Malawi, South Africa, and Tanzania, anticipates enrollment of 3900 high-risk females to study the efficacy and safety of TDF plus emtricitabine.18,21 Finally, the VOICE Study has the most aggressive enrollment plan of any study to date.18,19 The Microbicide Trials Network (MTN) and NIH co-sponsored trial anticipates enrollment of 4200 sexually active women to determine the safety and efficacy of TDF orally, TDF plus emtricitabine orally, and TDF vaginally. Both of these trials were originally scheduled to begin in 2008: FEM-PrEP in the third quarter and VOICE in the fourth quarter. Anticipated completion dates and supplemental information are delineated in Table 3.
CHALLENGES TO NAVIGATE
While the discovery of reliable methods to prevent HIV transmission is critical to stop the global spread of the disease, the road to this knowledge has proven difficult to navigate. Ethical concerns, patient recruitment, study design, and site requirements are just a few of the challenges that researchers and sponsors face when trying to implement a PrEP trial.
Barriers to Practice Implementation
Even if efficacy of PrEP therapy is established and ultimately approved by the FDA, the long term durability of preventative HIV treatment may be compromised as seen in Highly Active Antiretroviral Therapy (HAART) by emerging resistance due to patients non-adherence. Presently, most of the data that presumes PrEP therapy will not lead to resistance of future ARV therapy is generalized from the clinical findings of the ongoing Botswana trial.28 Ultimately, long-term follow-up is necessary in order to evaluate the safety and efficacy of PrEP. Additionally, strong clinical evidence shows that non-compliance or intermittent use of HAART quickly leads to viral mutation and resistance in standard HIV treatment.29-31 It is therefore scientifically reasonable that intermittent use of PrEP can put selective pressure on the viral strain that study participants carry, potentially leading to the development of mutation and resistance. These concerns have already led to the discontinuation of one trial.8
An increase in risky behavior practices is also of concern and elucidating the influence of PrEP use on behavior has been a focus of researchers. There have been numerous findings indicating that increased sexual risk taking, a resurgence of sexually transmitted infections, and an increased incidence of HIV among MSM has occurred globally following the introduction of HAART.32 Therefore, it is understandable that the potential for PrEP to encourage risky behavior is a serious concern. As PrEP gained notoriety in the media, anecdotal reports of its use among individuals increased – especially in the MSM population.33 This is of particular concern as it is being used in conjunction with club drugs in unmonitored, high-risk patients who may unknowingly seroconvert.33,34 The wealth of misinformation that exists surrounding the use of PrEP threatens to derail the future success of the therapy. However, this negative outcome may be attenuated if effective educational initiatives and behavioral therapy can be implemented to increase the use of barrier methods including condoms and decrease activities such as needle sharing.
Another pragmatic consideration with the implementation of PrEP is the cost for patients to obtain this type of therapy. There is ongoing debate as to whether biomedical prevention approaches are reasonable and if they should be considered for health insurance coverage.32 Insurers will need to determine if they will cover drugs such as TDF for non-FDA approved PrEP therapy as drugs are similarly covered for other off-label uses.32 Unless clinical trials provide enough evidence for medications to gain FDA approval with PrEP as an indication, most insurers will likely remain wary of undertaking such economic and legal responsibilities. The cost of PrEP per infection avoided should be lower than the estimated lifetime treatment cost of an HIV-infected individual in order for therapy to be considered cost-effective.14 This may be captured more substantially in populations where high and extremely high rates of risk behavior exist.
Source(s) of PrEP-targeted Antiretrovirals
The caution about resistance developing is especially warranted in PrEP use that takes place outside of controlled or study settings. As awareness of this novel, unapproved therapy spreads globally, the methods by which PrEP users obtain their medication will be of vital concern. Some physicians are writing prescriptions for the off-label use of HIV drugs for their patients.35 Other suppliers of PrEP may be friends or colleagues who take TDF as part of their HAART regimen. That scenario could create a domino effect in which neither the individual trying to employ PrEP nor the HIV positive friend is taking a sufficient dosage to be effective.
With the advent of Internet pharmacy, it is also possible that patients are purchasing HIV medications without the knowledge of healthcare professionals, seroconverting, and eliminating those drugs as future treatment options. The quality of antiretroviral drugs purchased from the Internet may also be of suspect quality and thus may not confer any protection – especially in light of the recent reports that up to 62% of all medicines purchased online are counterfeit or do not meet minimal quality standards.36 However, the lure of the Internet can be strong since Internet pricing for TDF or TDF plus emtricitabine can be substantially lower than that available from retail pharmacies, and many Internet pharmacy websites advertise these medications at up to 90% less than chain pharmacies.37-39 However, the quality of medications obtained via the Internet is not the only concern with this mode of acquisition. There is also the potential risk for various adverse effects and drug interactions in patients who are not appropriately managed.33
ROLE OF THE PHARMACIST
When confronted with questions regarding PrEP, pharmacists need to be able to make informed and appropriate recommendations to their patients about its safety, efficacy, and acquisition. Until additional clinical trials have been concluded, the safety and efficacy of PrEP remains to be established, and patients should carefully weigh the risks versus benefits of using an unproven regimen.
One area where pharmacists can make an essential intervention is in the process of patient acquisition of medications for PrEP. With medications of all types so readily available on the Internet without a prescription, acquiring PrEP medications has become much easier. Pharmacists should counsel patients about the inadvisability of obtaining medications from a disreputable source on the Internet. Patients should be made aware of the high percentage of counterfeit medications that are being sold by online pharmacies and cautioned about the dangers of obtaining medications that have not been subjected to the rigorous tracking requirements. Pharmacists can also direct patients who have a valid prescription to those Internet pharmacies that have received the National Association of Boards of Pharmacy (NABP) Verified Internet Pharmacy Practice Sites (VIPPS) designation of Recommended Internet Pharmacy.40 This designation indicates that the pharmacy is in compliance with the requirements of the state in which they operate, as well as all of the states to which they dispense medications. The NABP also provides a publicly accessible list of Internet pharmacy websites that are Not Recommended Sites for demonstrating a deficiency in one or more of five categories: 1) licensure and residency, 2) prescriptions, 3) patient privacy, 4) customer service, or 5) professional practices.
Pharmacists can also discuss the pitfalls of borrowing or sharing medications. Pharmacists should dissuade patients from these practices, which could lead to resistance and possible treatment failure on the part of the lender. In addition to the clinical complications that can arise from medication sharing, there are logistical problems as well. Many HIV patients obtain their medications through federal, state, or private funding sources.41 These programs typically do not allow patients to refill their prescriptions early or have policies in place for medication adherence requirements.
Another potential problem with PrEP use that lends itself well to pharmacist intervention is drug or disease state management. Many HIV medications, including those popularly used as PrEP, have clinically important drug interactions or interfere with certain disease states. For example, TDF and emtricitabine both carry a black box warning stating that the drugs should be used with extreme caution in patients who have risk factors for, or known, hepatitis or hepatic disease.22,23 These classes of medications can cause severe or fatal complications, such as lactic acidosis, in this patient population. Also, both TDF and emtricitabine have warnings about immune reconstitution syndrome (IRS) in patients on combination therapy, though the likelihood in this patient population is low. IRS is characterized by a severe inflammatory response in patients with latent or residual opportunistic infections including Mycobacterium avium complex, cytomegalovirus, Pneumocystis jiroveci pneumonia, or tuberculosis. Drug interactions should also be a concern for patients taking undocumented TDF or emtricitabine. Both interact with other medications commonly employed in the HIV population, including medications used to treat the disease itself and the opportunistic infections often seen in this patient group. Pharmacists should caution patients that if they are going to use PrEP, it is important that the pharmacist and physician are both aware of the practice so that it can be factored into the patients overall treatment plan.
Lastly, patients motivated by concern about their risk for infection are the ones asking questions about this type of prevention strategy. Pharmacists are in a prime position to educate their patients on traditional prevention strategies as well. Both sexual and IDU abstinence remain the most reliable methods of preventing transmission, although this approach is not a likely to be embraced. Therefore, educating patients on the use of barrier methods with the use of condoms made of latex, polyurethane, or other synthetic materials used consistently and correctly with lubricant during each and every sexual act is important. Additionally, to prevent injection-related transmission, abstaining from sharing injection equipment (e.g., syringes, needles, cookers, cottons, water) with other persons is the most reliable traditional method pharmacists currently have to help patients address their concern regarding HIV prevention.42
CONCLUSIONS
With a vaccine or cure currently out of reach, effective methods to help stem the spread of HIV are necessary if we are to have any hope of reining in the proliferation of the virus and slowing the global pandemic. While the potential for PrEP to make a positive global impact is promising, more extensive trials are necessary to establish its safety and efficacy. Pharmacists are well-positioned to play a key role in helping patients make choices about PrEP, managing their therapy, and developing policy with an eye towards the future.
CONFLICT OF INTEREST
No authors have any conflict of interest – financial or otherwise – in conjunction with this manuscript.
| References |
1. World Health Organization. UNAIDS. Global summary of the AIDS epidemic, December 2007. Available at:http://data.unaids.org/pub/EPISlides/2007/071118_epicore2007_slides_en.pdf (accessed October 17, 2007). [ Links ]
2. Panlilio AL, Cardo DM, Grohskopf LA, Heneine W, Ross CS; U.S. Public Health Service. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1-17. [ Links ]
3. Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, Cheever L, Johnson M, Paxton LA, Onorato IM, Greenberg AE; U.S. Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54(RR-2):1-20. [ Links ]
4. García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. [ Links ]
5. Marco M. Awareness and use of HIV pre-exposure prophylaxis (PrEP) are found to be low in San Francisco gay and bisexual men. AIDSmap News, 2006. Available at:www.aidsmap.com/en/news/656C2D2C-8629-4B08-8772-EAC5C04DDF71.asp (accessed April 3, 2008). [ Links ]
6. Singh JA, Mills EJ. The abandoned trials of pre-exposure prophylaxis for HIV: what went wrong? PLoS Med. 2005;2(9):e234. [ Links ]
7. Lange JM. We must not let protestors derail trials of pre-exposure prophylaxis for HIV. PLoS Med. 2005;2(9):e248. [ Links ]
8. Kresge KJ. AIDS Vaccine Bulletin. Treatment as prevention, May 2006, 4(5):1-4. Available at: www.iavireport.org/vax/VAXMay2006.asp (accessed July 11, 2008). [ Links ]
9. American Hospital Formulary Service Drug Information [Internet database]. Bethesda, MD: American Society of Health-System Pharmacists. Updated periodically. Available at: www.ashp.org/ahfs/index.cfm (accessed July 16, 2008). [ Links ]
10. Clinical Pharmacology [Internet database]. Tampa, FL: Gold Standard. Updated periodically. Available at: www.clinicalpharmacology.com (accessed July 16, 2008). [ Links ]
11. Lexi-Comp [Internet database]. Hudson, OH: Lexi-Comp, Inc. Updated periodically. Available at: www.lexi.com (accessed July 16, 2008). [ Links ]
12. Micromedex® Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Healthcare. Updated periodically. Available at: www.micromedex.com (accessed July 16, 2008). [ Links ]
13. Jackson JB, Barnett S, Piwowar-Manning E, Apuzzo L, Raines C, Hendrix C, Hamzeh F, Gallant J. A phase I/II study of nevirapine for pre-exposure prophylaxis of HIV-1 transmission in uninfected subjects at high risk. AIDS. 2003;17(4):547-553. [ Links ]
14. Derdelinckx I, Wainberg MA, Lange JM, Hill A, Halima Y, Boucher CA. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med. 2006;3(11):e454. [ Links ]
15. Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, Grant R, Clarke EE, Doh AS, Ridzon R, Jaffe HS, Cates W. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27. [ Links ]
16. CDC Fact Sheet. CDC trials of pre-exposure prophylaxis for HIV prevention, April 2007. Available at: www.cdc.gov/hiv/resources/Factsheets/PDF/prep.pdf (accessed June 7, 2008). [ Links ]
17. Grant R. The iPrEx study: safety, efficacy, behavior, and biology. Available at:www.hptn.org/web%20documents/PrEPMeetingMay2008/BobGranthptn2008may2.pdf (accessed June 7, 2008). [ Links ]
18. Prep Watch. Clinical trials. Ongoing and planned PrEP trials as of June 2008. Available at: www.prepwatch.org/pdf/Trials/PrEP_trials_table.pdf (accessed June 7, 2008). [ Links ]
19. Chirenje ZM, Marrazo J; Microbicide Trial Network. Phase 2B safety and effectiveness study of tenofovir 1% gel, tenofovir disoproxil fumarate tablet and emtricitabine/tenofovir disoproxil fumarate tablet for the prevention of HIV infection in women. Available at: www.mtnstopshiv.org/files/attachments/MTN-003%20Version%201%200%2022MAY2008.pdf (accessed July 10, 2008). [ Links ]
20. US National Institutes of Health Clinical Trial Registration. Pre-exposure prophylaxis to prevent HIV-1 acquisition within HIV-1 discordant couples (PartnersPrEP). Available at: www.clinicaltrials.gov/ct2/show/NCT00557245 (accessed July 10, 2008). [ Links ]
21. US National Institutes of Health Clinical Trial Registration. FEM-PrEP (Truvada®): study to assess the role of Truvada® in preventing HIV acquisition in women. Available at: www.clinicaltrials.gov/ct2/show/NCT00625404?term=fem-prep&rank=1 (accessed July 10, 2008). [ Links ]
22. Gilead Sciences, Inc. Viread (tenofovir disoproxil fumarate) package insert. Foster City, CA; 2008. [ Links ]
23. Gilead Sciences, Inc. Truvada (emtricitabine/tenofovir disoproxil fumarate) package insert. Foster City, CA; 2008. [ Links ]
24. Bristol-Myers Squibb & Gilead Sciences, LLC. Atripla (Efavirenz, emtricitabine, and tenofovir) package insert. Foster City, CA; 2006. [ Links ]
25. van 't Klooster G, Verloes R, Baert L, van Velsen F, Bouche MP, Spittaels K, Leempoels J, Williams P, Kraus G, and Wigerinck P. Long-acting TMC278, a parenteral depot formulation delivering therapeutic NNRTI concentrations in preclinical and clinical settings (abstract). Available at: http://www.retroconference.org/2008/Abstracts/31749.htm (accessed July 11, 2008). [ Links ]
26. Dumond J, Patterson K, Pecha A, Werner R, Andrews E, Damle B, Tressler R, Worsley J, Boggess K, and Kashuba A. Maraviroc (MVC) pharmacokinetics (PK) in blood plasma (BP), genital tract (GT) fluid and tissue in healthy female volunteers. (Abstract). Available at: www.retroconference.org/2008/Abstracts/33387.htm (accessed July 8, 2008). [ Links ]
27. Pfizer Inc. Selzentry (maraviroc) package insert. New York, NY; 2007. [ Links ]
28. Vissers DC, Voeten HA, Nagelkerke NJ, Habbema JD, de Vlas SJ.The impact of pre-exposure prophylaxis (PrEP) on HIV epidemics in Africa and India: A simulation study. PLoS ONE. 2008;3(5):e2077. [ Links ]
29. Maggiolo F, Airoldi M, Kleinloog HD, Callegaro A, Ravasio V, Arici C, Bombana E, Suter F. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8(5):282-292. [ Links ]
30. Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS, Montaner JS. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339-347. [ Links ]
31. Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, Kaplan AH. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41(3):315-322. [ Links ]
32. Szekeres G, Coates TJ, Frost S, Leibowitz A, Shoptaw S. Anticipating the efficacy of HIV pre-exposure prophylaxis (PrEP) and the needs of at-risk Californians. Center for HIV Identification, Prevention, and Treatment Services (CHIPTS). November 2004. Available at: http://chipts.ucla.edu/TEMPMAT/PrepReport/PrepReport1104.pdf (accessed July 18, 2008). [ Links ]
33. Liu AY, Grant R, Buchbinder SP. Preexposure prophylaxis for HIV: unproven promise and potential pitfalls. JAMA. 2006;296(7):863-865. [ Links ]
34. Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146(8):591-601. [ Links ]
35. Cohen J. Protect or disinhibit? New York Times Magazine. January 22, 2006. Available at: http://www.nytimes.com/2006/01/22/magazine/22wwln_essay.html (accessed July 2, 2008). [ Links ]
36. European Alliance for Access to Safe Medicines. The Counterfeiting Superhighway – the growing threat of online pharmacies. Available at:v35.pixelcms.com/ams/assets/312296678531/455_EAASM_counterfeiting report_020608.pdf (accessed July 4, 2008). [ Links ]
37. Medrx-one. Available at: www.medrx-one.com (accessed July 16, 2008). [ Links ]
38. Planet drugs direct. Available at: www.planetdrugsdirect.com (accessed July 16, 2008). [ Links ]
39. Indian Pharmaceutical Associations. International drug mart. Available at: www.internationaldrugmart.com (accessed July 16, 2008). [ Links ]
40. The National Association of Boards of Pharmacy. Available at: www.nabp.net (accessed July 11, 2008). [ Links ]
41. The Henry J. Kaiser Family Foundation. Insurance Status of AIDS Drug Assistance Program (ADAP) Clients, June 2007. Available at: www.statehealthfacts.org/comparetable.jsp?ind=542&cat=11(accessed July 11, 2008). [ Links ]
42. Centers for Disease Control and Prevention (CDC); Health Resources and Services Administration; National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2003;52(RR-12):1-24. [ Links ]