Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 no.3 Redondela jul./sep. 2009
| Original Research |
Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics
Encuesta a farmacéuticos y médicos sobre interacciones entre contraceptivos orales combinados y antibióticos de amplio espectro
Kelly P. MASTERS, Beth M. CARR.
| ABSTRACT Objective: To evaluate physician and pharmacist knowledge on potential drug interactions between combined oral contraceptives (COC) and broad-spectrum antibiotics and determine if any difference exists between responses. Key words: Drug Interactions. Anti-Bacterial Agents. Contraceptives, Oral, Combined. United States. | RESUMEN Objetivo: Evaluar el conocimiento de médicos y farmacéuticos sobre posibles interacciones medicamentosas entre contraceptivos orales combinados (COC) y antibióticos de amplio espectro, y determinar si existe diferencia entre ambas respuestas. Palabras clave: Interacciones medicamentosas. Antimicrobianos. Contraceptivos orales combinados. Estados Unidos. |
Kelly P. MASTERS. Pharm.D., BCPS. Assistant Professor of Pharmacy Practice. Bernard J. Dunn School of Pharmacy, Shenandoah University. Winchester, VA (United Stated).
Beth M. CARR. Pharm.D. Bernard J. Dunn School of Pharmacy, Shenandoah University. Winchester, VA (United Stated).
Received (first version): 26-Jan-2009
Accepted: 17-May-2009
INTRODUCTION
Anecdotal reports of combined oral contraceptive (COC) failure due to concurrent administration with broad-spectrum antibiotics have circulated the healthcare and general population for many years. Given the serious nature of this theoretical interaction, many healthcare practitioners now advocate additional contraceptive precautions when patients take concomitant COCs and antibiotics. Despite its widespread acceptance as a legitimate drug interaction, current recommendations state that a clinically significant interaction between COCs and broad-spectrum antibiotics does not exist.1,2
The authoritative textbook Contraceptive Technology states, "Broad-spectrum antibiotics such as amoxicillin and tetracycline do not reduce the efficacy of COCs. As a result, back-up methods should not be necessary".1 The interaction between COCs and broad-spectrum antibiotics is rated as a category 1 in the World Health Organization's Medical Eligibility Criteria for Contraceptive meaning that no restriction for the use of contraceptive method exists.2 A review of dermatology patient records found no significant difference in pregnancy rates between patients on COCs alone and patients on COCs and broad-spectrum antibiotics.3 Pharmacokinetic studies of antibiotics, other than rifampin, have documented the effect of broad-spectrum antibiotics on the levels of COCs and concluded that the antibiotics did not significantly decrease plasma steroid.4-8
Even with the lack of evidence for an interaction, many healthcare professionals still promote the theory of a drug interaction between COCs and broad-spectrum antibiotics and prescribe additional contraceptive precautions to their patients. Often, the precautions given to the patients are a complicated set of instructions that may result in patient confusion, non-compliance of either medication, or ultimately a high failure rate.
Pharmacists and physicians alike should be aware that studies show no decrease in efficacy of COCs when given with broad-spectrum antibiotics.3-8 Having an understanding of the data behind COC and antibiotics may help practitioners to better educate and counsel patients concerning this topic. The main objective of the survey was to evaluate the current knowledge of family practice physicians and pharmacists on the concomitant use of COCs and broad-spectrum antibiotics. A secondary objective was to investigate how practitioners handle the use of these 2 medications with their patients.
METHODS
Survey
A 3 question survey was designed to address the study objectives. The survey tool consisted of an anonymous, self-administered survey that was mailed to study participants. Prior to initiation of the study, the survey was distributed to six laypersons to assess the overall readability. Slight revisions were made based on the input received. The surveys were designed to be one page in length to increase participant response.
Study sample and data collection
The study sample was composed of 400 state-registered pharmacists in the state of Virginia and physicians practicing in the states of Virginia, West Virginia, and Maryland. A total of 200 community pharmacists, including both independent and chain store pharmacists, were selected to participate in the study. All retail pharmacies listed in the Yellow Pages local directory for the state of Virginia were randomized, using an Excel randomization function, to include 200 pharmacies. Surveys were mailed to each pharmacy requesting the attention of the pharmacist-in-charge. There were 200 Family Practice physicians who were selected to participate in the study. All Family Practice physicians who were registered with Optima Health, a Virginia-based health plan, were randomized, using an Excel randomization function, to include 200 physicians. In August 2007, all of the surveys were mailed to the study participants with a cover letter that described the details of the study. The surveys were sent with a self-addressed and stamped return envelope. Participants in the study had until the end of October to return the completed surveys.
The Shenandoah University Human Subjects Review Board approved the study protocol.
Data Analysis
Descriptive statistics were calculated to typify the study population in terms of gender, educational degree, and years in practice. Categorical data were reported as absolute numbers and percentages. Years in practice was categorized in the final analysis to determine if any differences existed in the respondents answers based on how long they had been out of school. It was hypothesized that those just recently out of their schooling may indicate that there is no drug interaction between COCs and broad-spectrum antibiotics than those further out. Pivot tables were designed to evaluate the survey responses from the participants. Contingency tables were formulated and Pearson chi-square analysis was used to assess the participants' responses, where appropriate. A P' value of less than 0.05 was considered significant. In cases where small cells (<5) were encountered a Fisher's exact test was performed. SPSS statistical package version 15.0 was used in the analysis of the data.
RESULTS
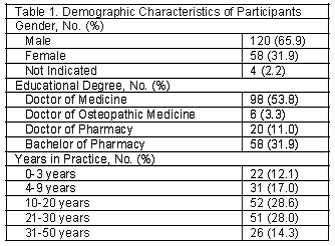
The demographic characteristics of the 182 respondents who returned completed mailed surveys are summarized in Table 1. Over 70% reported to have greater than 10 years of practice in their respective fields and 57.1% had medical degrees, either Doctor of Medicine or Doctor of Osteopathic Medicine, and 42.9% of the respondents were pharmacists, holding either a Bachelor of Pharmacy degree or Doctor of Pharmacy degree.
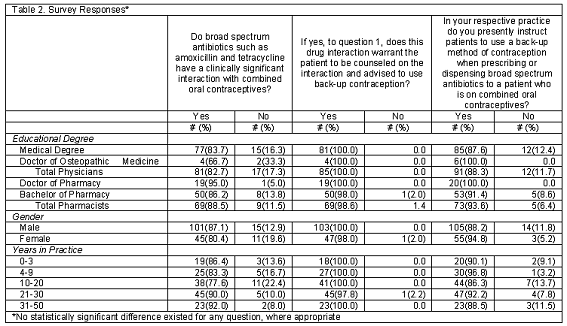
When responding to question one, "Do broad spectrum antibiotics such as amoxicillin and tetracycline have a clinically significant interaction with combined oral contraceptives," 82.7% of physicians and 88.5% of pharmacists stated, "yes". When analyzed separately there was no statistical difference in the responses to question one by individual degree between the physicians or the pharmacists. There was no statistical difference among male and female responses to question one in the survey, as well. Likewise, the number of years in practice for both professions did not cause a statistically significant difference in the responses (Table 2).
Question two of the survey asked the respondents who answered "yes" to question one if "the drug interaction warrants patients to be counseled on the interaction and advised to use back-up contraception?" All physicians who answered "yes" to question one also answered "yes" to question two indicating that the patient should be counseled to use a method of back-up contraception. Nearly 99% of the pharmacists also indicated that a back-up method of contraception should be used. Only 1 female respondent answered "no" to the counseling question and only 1 respondent when looked at by years in practice answered "no" to the question also.
Question three of the survey asked if respondents were currently instructing patients to use an alternative contraception method when prescribing or dispensing broad spectrum antibiotics to a patient who is also currently taking a COC. A greater percentage of pharmacists (93.4%) recommend to patients that they should use a back-up method of contraception compared to physicians (88.3%), No significant difference was shown to this question with regards to gender or years in practice.
DISCUSSION
Recommendations on the use of a back-up method of contraception for women taking concomitant oral antibiotics vary from resource to resource. A drug interaction check on Lexi-Comp ONLINETM rates the interaction with COC and penicillin as a B, indicating that no action is needed.9 Facts & Comparisons 4.0 also states that there is no interaction.10 Clinical Pharmacology rates the interaction as a Level 3, moderate and provides detailed information from studies concerning the interaction.11 Micromedex® Healthcare Series rates the same interaction as moderate with good documentation and states under clinical management that an additional form of birth control should be used.12 It is this variability which can lead healthcare professionals and patients confused about the most appropriate course of action.
The mechanism of action of the drug interaction between COCs and certain antibiotics is the direct result of alterations in pharmacokinetic parameters.13 Medications which can induce liver enzymes can also cause a reduction in ethinyl estradiol and progestins. These medications can include, but are not limited to, rifampicin, rifabutin, carbamazepine, griseofulvin, and many antiretroviral medications.13 Rifampin is a potent hepatic inducer of the cytochrome P-450 3A4 enzyme and ethinyl estradiol is a substrate of this enzyme as well.14 The increase in hepatic clearance of COC's by rifampin is so strong that COCs are contraindicated for 1 month after stopping rifampin.1 It is also well known that ethinyl estradiol undergoes enterohepatic recirculation once absorbed. In the large intestines, conjugates of ethinyl estradiol are broken down by hydrolytic enzymes which are released from colonic bacteria, these free (active) metabolites are then reabsorbed.12 Antibiotics which are non-liver enzyme inducers may reduce colonic bacteria and thus prevent the conversion of the ethinyl estradiol conjugates into active metabolites which are reabsorbed.12 A back up method of contraception has been recommended in the past for patients on COCs and antibiotics due to the enterohepatic recirculation mechanism. However, the authoritative textbook, Contraceptive Technology, states as a general rule a back-up method of contraception is not needed for women taking broad-spectrum antibiotics concomitantly with COCs.1 The rationale is that the progestin component of COCs is mainly responsible for the contraceptive benefit by thickening of the cervical mucus and since progestins do not undergo enterohepatic recirculation they are not affected by antibiotics.1 In addition to Contraceptive Technology, the World Health Organizations Medical Eligibility Criteria for contraception also states that there is no restriction on the use of broad-spectrum antibiotics and COCs.2 An additional reference, from the Council on Scientific Affairs of the American Medical Association states that the risk is small, however, emphasize there may be some interpatient variability and the use of a nonhormonal contraceptive method may be used if women are not comfortable with that risk.15
Several studies over the years have examined this theory. The effect of ampicillin on steroid levels was evaluated by Friedman, et al in the late 1970s.4 The study included 11 regularly menstruating women ages 21-39. Following one baseline cycle, subjects were instructed to take 50 ug of ethinyl estradiol (EE) and 1 mg of ethynodiol diacetate (Demulen®) for 2 consecutive cycles, 21 days on and 7 days off. Ampicillin 250 mg or placebo was taken 4 times a day from day 1 through day 16 of each study cycle. The subjects were studied for two consecutive menstrual cycles and results of the study found that there was no significant difference in levels of luteinizing hormone or follicle stimulating hormone between subjects. In addition, there was no significant difference in the levels of progesterone between the ampicillin and placebo treated cycles and the levels were all below luteal phase levels and inconsistent with ovulation.4
Co-trimoxazole (sulfamethoxazole 400mg-trimethoprim 80mg per tablet), another broad-spectrum antibiotic known to decrease bacteria in the large intestine, was investigated for its effect on oral contraceptive steroids in women.5 Nine women who had been taking long-term oral contraceptives with the triphasic preparation Trinordiol® (6 days on 30 ug EE and 50 ug levonorgestrel daily, 5 days on 40 ug EE and 75 ug levonorgestrel daily, and 10 days on 30 ug EE and 125 ug levonorgestreol daily) were studied. In the second cycle, subjects were given cotrimoxazole 2 tablets twice daily for 7 days starting on day 10 of the contraceptive. Study authors found no significant changes in the plasma concentration of levonorgestrel or progesterone between treatment and control cycle. There was a significant increase in EE levels that occurred during the cotrimoxazole therapy cycle. The authors concluded that it would be unlikely that a short-term course therapy with cotrimoxazole would result in a clinical problem in women taking COC.5
A prospective study involving 24 women between the ages of 18-35 years of age was conducted to evaluate the effect of doxycycline on serum levels of EE, norethindrone, and endogenous progesterone.6 The study consisted of a control and a treatment phase. All subjects had been taking a low-dose COC containing 1 mg norethindrone and 35 ug ethinyl estradiol (Ortho-Novum 1/35; Ortho Pharmaceutical Corp., Raritan, NJ) for at least 2 months prior to the study. During the treatment phase the subjects continued to take the COC and were also given doxycycline 100mg by mouth twice daily for 7 days beginning on day 14 of the cycle. There was no significant difference in plasma levels of EE, norethindrone, or progesterone between the control and treatment phases.6 A similar study using tetracycline 500mg every 6 hours also showed that oral contraceptive levels are not decreased by concomitant ingestion of tetracycline.7
A double-blind, placebo-controlled, randomized, crossover trial of 24 healthy female volunteers taking a COC were given ciprofloxacin on days 8-17 for 2 consecutive cycles. The main objective of the study was to show that plasma levels of EE during ciprofloxacin treatment were identical to those seen during placebo treatment. In addition to measuring plasma levels of EE and sex hormone binding globulin (SHBG), all subjects had a transvaginal ultrasound performed at days 8, 10, 12, and 14 to measure the increase in the follicular diameter. All patients had progesterone levels <2 ng/mL indicating the absence of ovulation. Based on the serum estradiol levels no patients had indicators of ovarian activity. The levels of SHBG were similar in both the treatment and placebo groups. Two patients were potentially ovulatory based on follicle diameter size; however both occurred in the placebo treatment group. Final conclusions of the study indicate that ciprofloxacin does not alter ovarian activity when co-administered with a low dose contraceptive.8
Despite evidence disproving the interaction between COC's and broad spectrum antibiotics, both physicians and pharmacists continue to recommend a back up method of contraception to patients who are concomitantly taking a broad-spectrum antibiotic and COC. Potential reasons behind practitioners recommending additional contraception to their patients may include: contradicting information in drug information resources, lack of information/awareness, mistrust in available data/literature, concern for a woman's desire to prevent pregnancy, or for liability issues. Because practitioners of the health sciences should always strive to present accurate and truthful information; it would prove beneficial to provide these practitioners with further education on the current evidence on drug interactions with COCs.
In addition to the obligation that health care practitioners have to present information to their patients based on current scientific evidence, there are other potential consequences that may occur as a result of recommending a back up method of contraception to patients concomitantly taking COCs and a broad-spectrum antibiotic. Patients may become confused with instructions on how long and when to use a back-up method of contraception. This confusion can potentially lead to patients discontinuing their COCs altogether which ultimately increases the risk of pregnancy. In addition, patients taking COCs for reasons other than pregnancy prevention (eg. acne, PMS, etc.) may stop the COCs which may affect some quality of life issues. The confusion could also lead to patients not taking or completing the full course of therapy of the broad-spectrum antibiotic, which could result in many negative, health related outcomes. Furthermore, female patients who are concomitantly taking a long-term, broad-spectrum antibiotic and COCs may become discouraged when told their COCs are less effective due to a drug interaction. These patients may opt to discontinue the use of their COCs and opt for a less effective form of birth control, thereby increasing their chances of pregnancy. By being aware of the data regarding COCs and antibiotics, practitioners will be able to better counsel their patients on the true risks and benefits that exist when taking the two drugs concomitantly. Additionally, with more education, practitioners will apt to provide accurate information to patients should the patients be concerned that they may get pregnant if they do not use a back up method of contraception.
There were several limitations of this study. First, a post-hoc power analysis included power values ranging from 0.14-0.34. A difference between physicians and pharmacists may not have been detected due to the low power. Despite the low power, the survey results highlight an important gap in knowledge of many practitioners on the topic. The surveys were mailed only to practitioners practicing in the state of Virginia with a few surveys sent to Maryland and West Virginia. This study population is not representative of all physicians and pharmacists. In addition, the surveys merely asked about broad-spectrum antibiotics. Since no specific antibiotics were provided, practitioners may have erred on the side of caution and stated that they do recommend a back-up method of contraception. The study could be expanded upon by stating specific antibiotics so as to avoid any confusion with the terminology "broad-spectrum antibiotics." In addition, upon further review, question 2 of the survey may have promoted some bias in that it did not allow the respondent to indicate the rate of instruction (e.g. 50% of the time, always, etc). Another limitation of the study is that it is unknown what type of back-up method is generally recommended and for how long it is recommended to be used.
The current study also did not provide an area for practitioners to discuss the mechanism of the drug interaction. It may be interesting to evaluate what physicians versus pharmacists believe is the exact mechanism of action of the drug interaction between broad-spectrum antibiotics and COCs.
CONCLUSIONS
Physicians and pharmacists believe that broad-spectrum antibiotics decrease the effectiveness of COCs and commonly instruct their patients to use a back-up method of contraception when prescribing or dispensing broad spectrum antibiotics to these women. Due to the fact that leading experts are recommending that a back-up method is generally not necessary when prescribing broad-spectrum antibiotics, it is important that more education be provided to practitioners and patients regarding the absence of a proven interaction between COCs and antibiotics to avoid the confusion surrounding this important topic. Although many practitioners may continue to recommend an alternative contraceptive during antibiotic use, should a patient choose not to use a back-up method the practitioner can counsel the patient on the low risk involved of her becoming pregnant.
CONFLICT OF INTEREST
The project was funded by an internal research grant from the Bernard J. Dunn School of Pharmacy, Shenandoah University.
The authors declare no conflicts of interest or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
| References |
1. Hatcher R, Trussel J, Nelson AL, Cates W, Stewart FH, Kowal D. Contraceptive Technology, 19th ed. New York: Ardent Media; 2007. [ Links ]
2. WHO Department of Reproductive Health and Research. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. [Cited: September 5, 2007]. Available from: http://www.who.int/reproductive-health/publications/mec/index.htm [ Links ]
3. Helms S, Bredle D, Zajic J, Jarjoura D, Brodell RT, Krishnarao I. Oral Contraceptive failure rates and oral antibiotics. J Am Acad Dermatol. 1997;365:705-710. [ Links ]
4. Friedman C, Huneke A, Kim M, Powell J. The effect of ampicillin on oral contraceptive effectiveness. Obstet Gynecol. 1980;55:33-37. [ Links ]
5. Grimmer SFM, Allen WL, Back DJ, Breckenridge AM, Orme M, Tija J. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59. [ Links ]
6. Nely J, Abate M, Swinker M, D'Angio R. The effect of doxycycline on serum levels of ethinyl estradiol, norethinderone and endogenous progesterone. Obstet Gynecol. 1991;77:416-420. [ Links ]
7. Murphy AA, Zacur HA, Charache P, Burkman RT. The effect of tetracycline on levels of oral contraceptives. Am J Obstet Gynecol. 1991;164:28-33. [ Links ]
8. Scholten PC, Droppert RM, Zwinkels MGJ, Moesker HL, Nauta JJP, Hoepelman IM. No Interaction between Ciprofloxacin and an Oral Contraceptive. Antimicrob Agents Chermother. 1998 Dec; 42:3266-3268. [ Links ]
9. Anon. Lexi-comp ONLINE Interaction Monograph (database on the internet). Lexi-Comp Online Lexi-Interact 2008, Lexi-Comp. (cited 2008 July 8). Available from: http://online.lexi.com [ Links ]
10. Anon. In: Tatro DS, ed. Drug Interaction Facts. Facts & Comparisons. Philadelphia, PA; Wolters Kluwer Health, Inc.© 2008. (cited 2008 July 8). Available from: http://clinicalresource.ovid.com/clinicalresource/re/drugHome [ Links ]
11. Anon. Drug Interaction Report for Healthcare Professionals. In: Reents S, Hochadel MA, Vieson KJ, et al. Clinical Pharmacology 2008, Gold Standard Multimedia Inc.(cited 2008 July 8). Available from: http://cp.gsm.com [ Links ]
12. Micromedex® Healthcare Series (database on the internet): Thomson Micromedex, Greenwood Village, Colorado. (cited 2008 July 8). Available from: http://www.thomsonhc.com/home/dispatch [ Links ]
13. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. FFPRHC Guidance (April 2005). Drug Interactions with Hormonal Contraception. J Fam Plann Reprod Health Care 2005:31(2):139-150. [ Links ]
14. Hansten PD, Horn JR. The Top 100 Drug Interactions: A Guide to Patient Management, 2004 ed. Edmonds, WA:H & H Publications;2004. [ Links ]
15. Dickinson BD, Altman RD, Nielsen NH, Sterling ML. Drug Interactions Between Oral Contraceptives and Antibiotics. Obstet Gynecol 2001;98:853-860. [ Links ]