Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.6 no.4 Madrid nov./dic. 2014
https://dx.doi.org/10.4321/S1889-836X2014000400006
Advances in the study of the mechanisms involved in the modulation of the expression of sclerostin in human cells
Avances en el estudio de los mecanismos involucrados en la modulación de la expresión de esclerostina en células humanas
Delgado-Calle J.1, Pérez-Campo F.M.2 and Riancho J.A.2
1 Departamento de Anatomía y Biología Celular - Facultad de Medicina de Indiana - Centro Médico de Administración de Veteranos Roudebush - Indianapolis (EE.UU. )
2 Departamento de Medicina Interna - Hospital Universitario Marqués de Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
Work rewarded with the scholarship investigation FEIOMM 2011.
SUMMARY
Sclerostin plays an important role in the regulation of bone metabolism, as is shown in the dramatic changes in bone mass which occur when its activity is inhibited by means of monoclonal antibodies. However, the mechanisms which regulate its expression are still not well-understood. Various studies have shown an association between polymorphisms of the SOST gene promoter (which codes for sclerostin) and bone mineral density. Also, the degree of methylation of a CpG island near the start of the transcription is associated with marked changes in the expression of the gene. Therefore, it appears that the production of sclerostin is influenced by both genetic and epigenetic mechanisms, in addition to other hormonal and mechanical factors. A greater knowledge of these mechanisms would not only contribute to a better understanding of bone biology, but could open up new therapeutic opportunities.
Key words: sclerostin, SOST, methylation, epigenetic.
RESUMEN
Objetivos: La esclerostina desempeña un papel importante en la regulación del metabolismo óseo, como queda demostrado por los cambios dramáticos en la masa ósea que se producen cuando se inhibe su actividad mediante anticuerpos monoclonales. Sin embargo, aún no se conocen bien los mecanismos que regulan su expresión. Varios estudios han demostrado una asociación entre polimorfismos del promotor del gen SOST (que codifica la esclerostina) y la densidad mineral ósea. Asimismo, el grado de metilación de una isla CpG próxima al inicio de la transcripción se asocia a cambios marcados en la expresión del gen. Por tanto, parece que la producción de esclerostina está influida tanto por mecanismos genéticos como epigenéticos, además de otros factores hormonales y mecánicos. Un mejor conocimiento de los mismos no sólo contribuirá a entender mejor la biología ósea, sino que puede abrir nuevas oportunidades terapéuticas.
Palabras clave: esclerostina, SOST, metilación, epigenética.
Introduction
Sclerostin is a protein coded by the SOST gene. This protein is secreted specifically by the osteocytes and has a negative effect on bone formation, through the inhibition of the Wnt canonical pathway1. The inhibition of this pathway has profound consequences for the activity of the osteoblasts; specifically, their differentiation is inhibited and their apoptosis induced2,3. The importance of sclerostin in bone biology has been seen in the description of cases of mutations of the SOST gene in humans which provoke an altered bone phenotype, with an increased bone mass4,5. On the other hand, the inhibition of sclerostin through the use of neutralising antibodies has been demonstrated to have a powerful anabolic effect in bone, both in animals and in humans6,7.
Although the significance of sclerostin in bone homeostasis appears indubitable, there are various aspects of its biology which still remain unknown. Some of the least-known aspects are the factors which regulate the expression of sclerostin and the mechanisms involved. For example, it is not known why solely the osteocytes, and not other osteoblast line cells, are capable of expressing sclerostin. Possibly even more intriguing is the fact that there are osteocytes within the bone producing sclerostin, while others located within a few microns of them do not8.
In some experimental models various effectors have been identified which are capable of regulating levels of sclerostin. On the one hand, among the positive effectors are the bone morphogenetic proteins (BMPs)9 or the combined action of the tumor necrosis factor (TNF) and the tumour necrosis factor-like weak inducer of apoptosis (TWEAK)10. On the other, notable among the negative regulators are parathyroid hormone (PTH)11,12, prostaglandin E2 (PGE2)12 and mechanical load, this last factor being of special significance due to the role which this type of stimulus has on bone homeostasis13. Unfortunately, many of these experiments have been carried out in murine models and it remains to be seen to what extent they are transferable to human bone. Furthermore, although the effects of these factors have been described, the molecular mechanisms which underlie their effects on the expression of SOST have hardly been identified.
One of the obstacles which researchers are encountering when studying the regulation of sclerostin production is the absence of systems in which this gene is actively expressed. Currently, there are no human osteocyte lines available. The generation of some murine lines has been reported, but in spite of the fact that these show some of the characteristic phenotypes of osteocytes, their production of sclerostin is barely detectable. It would therefore of great interest to find a good system in which the factors involved in the regulation of the expression of this gene may be identified.
Curiously, not the entire promoter sequence for the SOST gene is preserved between species, which suggests that the regulation may differ as a function of the species. This is still more evidence of the necessity of developing human models. Some works suggest that region 5' of the gene would have two sections: one, close to the start of the transcription, which shows marked transcriptional activity, and the other, situated at some 1,000 base pairs' distance from the start of the transcription, which could have an inhibitory effect14. On the other hand, it is interesting to note that various groups, including ours, have shown an association between some polymorphisms located in the 5' region of the gene and bone mineral density (BMD)15,16. In the same vein, in genome-wide association studies (GWAS) some polymorphisms of a nucleotide (SNPs) have been found near this gene associated with BMD17. This suggests that these polymorphisms may have a functional impact and modulate the expression of the gene, but actually, it is not known if this really is the case or what would be the molecular mechanisms involved.
Given the importance attributed to sclerostin in bone formation, the identification of the molecular mechanisms which regulate its levels could open new areas of investigation in bone biology, and perhaps help to identify new therapeutic targets related to the inhibition of its production, which would have an anabolic effect on bone. Furthermore, the validation of new models of cells of human origin in which it would be possible to study these mechanisms could be crucial for the advancement of the understanding of the regulation of sclerostin. In this article we briefly review some recent results from our laboratory and those of other researchers to shed some light on these questions.
DNA methylation and the regulation of gene expression
A good number of the cytosines of mammalian DNA are methylated, especially when they are followed by a guanine, which is to say, when forming CG dinucleotides (often also known as CpGs, the "p” indicating the phosphate group which links the two bases). It is supposed that the methylation brings stability to the DNA and avoids "transcriptional noise” in the background. There are zones of DNA, called "CpG islands”, which have a particular behaviour. These islands consist of regions of a few hundred nucleotides which are especially rich in CpG and which are found frequently in the promoter regions of many genes. In recent years it has been shown that the level of methylation in these CpG islands (and in the adjacent regions, called "CpG island shores”) plays an important role in the regulation of the expression of many genes. In general, when the CpG of the promoter regions is highly methylated, the transcription of DNA to RNA is repressed, and, as a consequence, the levels of the protein for which the gene codes are reduced. Inversely, the demethylation of the promoter tends to be associated with the active transcription of the gene.
There are many molecular mechanisms involved in the regulation of gene expression through changes in methylation, of which only some are known. Thus, for example, the methylation of DNA may impede its bonding with some activating transcription factors. On the other hand, the methylated regions attract some proteins which bond specifically to these methylated regions. This is the case with MeCP2, the binding protein for methylated CpG18. However, it should also be taken into account that the methylation of DNA acts in combination with other epigenetic mechanisms, specifically with the postranslational modifications of the histones. In fact, when MeCP2 bonds to DNA, it recruits other proteins, such as HDACs (histone deacetylases) which modify the tails of the histones near this region. Together, these modifications contribute to the modulation of gene expression. For example, the highest levels of histone acetylation are usually associated with an activation of transcription; while, to the contrary, the methylation of certain lysines present in the histones is associated with gene repression19.
The patterns of DNA methylation are transmitted through mitosis, which means that they are inherited from the cell which divides into two daughter cells. In this process an essential role is played by a family of enzymes called DNA-methyltransferases (DNMTs), in particular type 120,21.
DNA methylation can be a passive phenomenon, which is to say, it may appear during some cell divisions if the DNMTs do not perform their function of the remethylation the DNA daughter chains. But demethylation may also be an active process. This is to suggest that it is possible that some regions of DNA are demethylated without the necessity of cell division and the consequent replication of DNA having taken place. The process by which active demethylation occurs is not well understood, but the enzyme GADD45 and the conversion of the methylcytosines to hydromethylcytosines appears to play a special role in it19,22,23. In addition, its true significance in tissue homeostasis is also not well known. Nevertheless, it has been suggested that this process could be involved in osteoblast differentiation24.
Our group has demonstrated that methylation and demethylation of some genes plays an essential role in variations in the patterns of gene expression which occur during the different stages of the differentiation of the osteoblast-lineage cells. For example, using the technique of laser assisted microdissection and subsequent DNA analysis of the cells thus captured, we have confirmed that during the step from osteoblasts to osteocytes there occurs a marked reduction in the methylation of the SOST gene promoter. Differently from that which occurs in the case of osteoblasts, this is a necessary requisite for osteoclasts to be able to synthesise sclerostin25. Other genes involved in the biology of the skeleton are also regulated, in part, through the level of methylation of their promoters. This is the case, for example, with osteoprotogerin, the ligand of RANK (RANKL), alkaline phosphatase, osterix or estrogen receptor26-28.
DNA demethylation as experimental tool
The changes in the methylation of the CpG islands are very powerful regulation mechanisms. They are possibly not involved in the fine regulation of gene expression, but act as a type of molecular "interrupter” which starts and stops gene transcription. Once the demethylation allows transcription, other mechanisms, (humoral, physical, etc.) will be responsible for adjusting precisely the gene expression, in response to what is required at that moment29.
The regulatory power of the mechanisms linked to methylation are shown in certain experiments in which DNA methylation is pharmacologically induced. For this, nucleotide analogs are often used, such as azacytidine and deoxy-azacytidine (or decytabine) which inhibit the activity of the DNMTs. Thus we have been able to demonstrate that the incubation of different types of cell with decytabine strongly induces the expression of sclerostin even when in normal conditions these cells do not express the gene25.
This phenomenon has, furthermore, an interesting practical repercussion, in that it facilitates the study of the mechanisms which modulate the expression of sclerostin. Given that there are no human osteocyte lineages, or techniques to isolate viable osteocytes from human bone, it is complicated to explore the regulatory mechanisms for this gene in humans. Although there are different immortalised osteoblast lineages, and it is relatively easy to obtain osteoblasts from bone biopsies, these cells do not express sclerostin. However, the demethylation of its promoter with decytabine induces the expression of this gene, thus functioning, at least in theory, as an experimental model to analyse the physical and chemical factors involved in its regulation.But for this model to be really useful the osteoblasts should have a pattern of response to different stimuli similar to that of the osteocytes in in vivo animal experimental models30,31.
In fact, this appears to be the case. The results which we have obtained with this model of osteoblasts treated with decytabine have confirmed the inhibitory effect of PTH and the stimulatory effect of the BMPs (bone morphogenetic proteins) on the expression of SOST32. We have also been able to confirm that the osteoblasts treated with decytabine maintain their response not only to humoral factors but also to mechanical stimuli.When these cells are subject to a pulsating flow of the culture medium (which simulates the stimulus of the osteocyte membranes by the liquid present in the lacunae and canaliculi of the bone) a series of biochemical responses is induced, notable among which is the induction of nitric oxide synthase (NOS), with its consequent accumulation in the medium. This response is maintained in the osteoblasts pretreated with decytabine. Furthermore, in these cultures it is possible to confirm that mechanical stimulus induces a reduction in the expression of sclerostin, in line with that demonstrated in in vivo experimental models13,33. The later experiments with nitric oxide inhibitors and donors have enabled the confirmation that nitric oxide synthesis really is involved in the inhibitor effect of SOST induced by mechanical stimulation34.
SOST gene promoter, sclerostin and bone mass
Various studies of candidate genes and also those of genome association (GWAS) have found polymorphisms of the SOST gene associated with bone mineral density15,35. Hence, we have proved that women who are homozygous for the minor allele (G) of SNP rs851054, situated in promoter region 5' of the gene, have a BMD significantly lower than women with other genotypes. This suggest that this polymorphism may provoke differences in transcriptional activity as a function of the allele which is present. To explore further the mechanisms involved, we cloned the entire SOST promoter region (positions -1440/+30 in relation to the transcription start site or TSS) and confirmed its transcriptional activity in a luciferase reporter vector, after its transfection in different types of cell. The cloning of various regions of this fragment allowed us to confirm that the most active region appears to be in the first 500 nucleotides. In fact, the transcriptional activity of the vectors with insertion in the region -580/+30 is somewhat greater than that of the complete regions (-1440/+30). Contrarily, the most distal region(-1440/1030) is not active, while the intermediate region (-1032/-571) has a certain degree of activity, although clearly lower than that of the complete region or the region nearest to the TSS.Also, we found that BMP2 increases the transcriptional activity of these constructions, while PTH has no effect, which is in accordance with those studies which show that the effect of this hormone is mediated by an enhancer region located in several thousand base pairs32.
On the other hand, from the genomic DNA of individuals for various polymorphisms frequent in the SOST promoter (rs801054 and rs801056), we cloned the promoter regions with each of the possible alleles for these polymorphisms in luciferase reporter vectors. We then analyzed its transcriptional activity after transfection into various osteoblast-type lineages. However, the differences in activity of the difference alleles were small (data not published). This suggests that the demonstrated association of these alleles with bone mineral density should be measured by indirect mechanisms which are not reproduced in these experimental models. Among these should be considered: certain factors, physical or humoral, present in vivo but not in vitro; the interaction of other cell elements in the bone microenvironment; or complex actions which involve the three dimensional structure of chromatin and the involvement of other distant regions of the DNA. An obvious candidate is called the Van Buchem region, situated several thousand gene bases away, and in which have been described regulatory regions (such as that called ECR5), which appear to mediate the response to some factors, PTH in particular36,37.
Conclusion
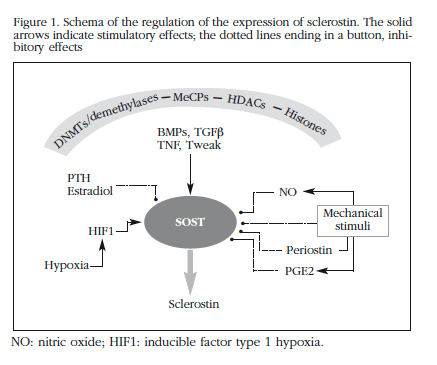
Sclerostin plays a significant role in the regulation of bone metabolism, as is demonstrated in the dramatic changes in bone mass which occur when its activity is inhibited by means of monoclonal antibodies38,39. However, knowledge of the mechanisms which regulate their expression is still incomplete. Nevertheless, in recent years new data have been generated which allow one to sketch out, albeit schematically, some of the factors and pathways involved (Figure 1).
This work and the experiments which are mentioned have also been carried out with the help of research grants from ISCIII (PI12/615) and with financial support from IFIMAV-IDIVAL.
The authors have no conflicts of interest in relation to this work.
![]() Correspondence:
Correspondence:
José A. Riancho Moral
Departamento de Medicina Interna
Hospital U. Marqués de Valdecilla
39011 Santander (Spain)
E-mail: jose.riancho@unican.es
Date of receipt: 18/08/2014
Date of acceptance: 15/10/2014
Bibliography
1. van Bezooijen RL, Roelen BA, Visser A, Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004;199:805-14. [ Links ]
2. Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 2004;35:828-35. [ Links ]
3. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 2005;19(13):1842-4. [ Links ]
4. Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 2001;10:537-43. [ Links ]
5. Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 2001;68:577-89. [ Links ]
6. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 2011;26:19-26. [ Links ]
7. Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res 2010;25:2412-8. [ Links ]
8. Delgado-Calle J, Arozamena J, Garcia-Renedo R, Garcia-Ibarbia C, Pascual-Carra MA, Gonzalez-Macias J, et al. Osteocyte deficiency in hip fractures. Calcif Tissue Int 2011;89:327-34. [ Links ]
9. Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, et al. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res 2010;25:200-10. [ Links ]
10. Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, et al. Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res 2009;24:1434-49. [ Links ]
11. Miniati M, Pistolesi M, Marini C, Di Ricco G, Formichi B, Prediletto R, et al. Value of perfusion lung scan in the diagnosis of pulmonary embolism: results of the prospective investigative study of acute pulmonary embolism (PISA-PED). Am J Respir Crit Care Med 1996;154:1387-93. [ Links ]
12. Genetos DC, Yellowley CE, Loots GG. Prostaglandin E(2) Signals Through PTGER2 to Regulate Sclerostin Expression. PLoS ONE 2011;6:e17772. [ Links ]
13. Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact 2006;6:354. [ Links ]
14. Sevetson B, Taylor S, Pan Y. Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST). J Biol Chem 2004;279:13849-58. [ Links ]
15. Valero C, Zarrabeitia MT, Hernandez JL, Pineda B, Cano A, Garcia-Perez MA, et al. Relationship of sclerostin and secreted frizzled protein polymorphisms with bone mineral density: an association study with replication in postmenopausal women. Menopause 2011;18:802-7. [ Links ]
16. Huang QY, Li GH, Kung AW. The -9247 T/C polymorphism in the SOST upstream regulatory region that potentially affects C/EBPalpha and FOXA1 binding is associated with osteoporosis. Bone 2009;45:289-94. [ Links ]
17. Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 2009;151:528-37. [ Links ]
18. Reddington JP, Pennings S, Meehan RR. Non-canonical functions of the DNA methylome in gene regulation. Biochem J 2013;451:13-23. [ Links ]
19. Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet 2012;13:7-13. [ Links ]
20. Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing Res Rev 2009;8:268-76. [ Links ]
21. Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol 2014;4:80. [ Links ]
22. Niehrs C, Schafer A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol 2012;22:220-7. [ Links ]
23. Pfeifer GP, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 2013;6:10. [ Links ]
24. Zhang RP, Shao JZ, Xiang LX. GADD45A protein plays an essential role in active DNA demethylation during terminal osteogenic differentiation of adipose-derived mesenchymal stem cells. J Biol Chem 2011;286:41083-94. [ Links ]
25. Delgado-Calle J, Sanudo C, Bolado A, Fernandez AF, Arozamena J, Pascual-Carra MA, et al. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J Bone Miner Res 2012;27:926-37. [ Links ]
26. Vrtacnik P, Marc J, Ostanek B. Epigenetic mechanisms in bone. Clin Chem Lab Med 2014;52:589-608. [ Links ]
27. Delgado-Calle J, Sanudo C, Sanchez-Verde L, Garcia-Renedo RJ, Arozamena J, Riancho JA. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone 2011;49:830-8. [ Links ]
28. Delgado-Calle J, Sanudo C, Fernandez AF, Garcia-Renedo R, Fraga MF, Riancho JA. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics 2012;7:83-91. [ Links ]
29. Delgado-Calle J, Garmilla P, Riancho JA. Do epigenetic marks govern bone mass and homeostasis? Curr Genomics 2012;13:252-63. [ Links ]
30. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008;283:5866-75. [ Links ]
31. Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di Vito M, et al. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 2007;38:261-9. [ Links ]
32. Delgado-Calle J, Arozamena J, Perez-Lopez J, Bolado-Carrancio A, Sanudo C, Agudo G, et al. Role of BMPs in the regulation of sclerostin as revealed by an epigenetic modifier of human bone cells. Mol Cell Endocrinol 2013;369:27-34. [ Links ]
33. Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 2009;24:1651-61. [ Links ]
34. Delgado-Calle J, Riancho JA, Klein-Nulend J. Nitric oxide is involved in the down-regulation of SOST expression induced by mechanical loading. Calcif Tissue Int 2014;94:414-22. [ Links ]
35. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491-501. [ Links ]
36. Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 2007;22:1957-67. [ Links ]
37. Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 2005;15:928-35. [ Links ]
38. McClung MR, Grauer A, boonen s, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412-20. [ Links ]
39. McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 2014;29:935-43. [ Links ]











 texto en
texto en