Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.7 no.4 Madrid nov./dic. 2015
https://dx.doi.org/10.4321/S1889-836X2015000400003
The VEGF (VEGFR2) 2 receptor and PTH (PTH1R) 1 receptor act as mediators in the anti-apoptotic response to mechanical stimulus in MLO-Y4 osteocyte-like cell
El receptor 2 de VEGF (VEGFR2) y el receptor 1 de la PTH (PTH1R) actúan como mediadores de la respuesta anti-apoptótica al estímulo mecánico en las células osteocíticas MLO-Y4
Maycas M.1, Fernández de Castro L.2, Bravo B.2, García de Durango C.2, Forriol F.2, R. Gortázar A.2, Esbrit P.1
1 Laboratorio de Metabolismo Mineral y Óseo - Instituto de Investigación Sanitaria (IIS)-Fundación Jiménez Díaz - UAM - Madrid (España)
2 IMMA-Facultad de Medicina Universidad San Pablo-CEU - Madrid (España)
Fellow work to attend the 34th Congress of the ASBMR (Minneapolis, 2012).
This work was funded by the Institute of Health Carlos III (PI050117, PI080922, PI11/00449, RD06/0013/1002 and RD12/0043/0008), the European Regional Development Fund (ERDF) and the University San Pablo CEU (Pre-competitive funding Santander-CEU).
SUMMARY
Mechanical stimulation plays a crucial role in bone mineral maintenance. This stimulation prevents osteocyte apoptosis by a mechanism that involves β-catenin accumulation and nuclear translocation of extracellular-signal-regulated kinases (ERKs). The vascular endothelial growth factor (VEGF) and parathyroid hormone-related protein (PTHrP) modulate bone formation, although their interaction with osteocytes is unknown. In this paper we have considered the possible role of VEGF (VEGFR2) 2 receptor and PTH (PTH1R) type 1 receptor in the anti-apoptotic response to mechanical stimulation of MLO-Y4 osteocyte-like cells. The cells were subjected to mechanical stress by laminar fluid flow (10 min, 10 dinas/cm2) or hypotonic shock (240 mOsm, 1h), or stimulated with VEGF165 or PTHrP (1-36). We also compared the effects of overexpressed VEGFR2 and mechanical stimulation of these cells. Mechanical stimulation, VEGF165 or PTHrP (1-36)stimulated cellular viability and β-catenin stabilization in a similar manner, associated with its localization in the membrane. Mechanical stimulation increased PTH1R presence in the membrane. VEGFR2 inhibition as well as the PTHrP (7-34) antagonist reduced these effects. On the other hand, VEGFR2 overexpression in MLO-Y4 cells mimicked the mechanical stimulation effect on β-catenin and cellular viability. Our findings support a functional role for both systems, VEGF/VEGFR2 and PTHrP/PTH1R, in the early response to mechanical stimulation in promoting osteocyte-like viability.
Key words: PTH1R, VEGFR2, mechanical stimulation, β-catenin, apoptosis.
RESUMEN
La estimulación mecánica juega un papel fundamental en el mantenimiento de la masa ósea. Dicha estimulación previene la apoptosis de los osteocitos por un mecanismo que implica la acumulación de β-catenina y la translocación nuclear de quinasas reguladas por señales extracelulares (ERK). El factor de crecimiento del endotelio vascular (VEGF) y la proteína relacionada con la parathormona (PTHrP) modulan la formación ósea, aunque su interacción con los osteocitos es desconocida. En el presente estudio hemos evaluado el posible papel del receptor 2 del VEGF (VEGFR2) y del receptor tipo 1 de PTH (PTH1R) en la respuesta anti-apoptótica a la estimulación mecánica en células osteocíticas MLO-Y4. Las células se sometieron a estrés mecánico por flujo laminar de fluido (10 min, 10 dinas/cm2) o choque hipotónico (240 mOsm, 1h), o estimuladas con VEGF165 o PTHrP (1-36). Además, comparamos los efectos de sobre-expresar VEGFR2 y el estímulo mecánico en estas células. La estimulación mecánica, el VEGF165 o la PTHrP (1-36), de manera similar, estimularon la viabilidad celular y la estabilización de β-catenina, relacionada con su localización en la membrana. Además, la estimulación mecánica aumentó la presencia del PTH1R en la membrana. La inhibición del VEGFR2 así como el antagonista PTHrP (7-34) disminuyeron estos efectos. Por otro lado, la sobre-expresión del VEGFR2 en las células MLO-Y4 mimetizó el efecto del estímulo mecánico sobre la β-catenina y la viabilidad celular. Estos hallazgos apoyan un papel funcional de ambos sistemas, VEGF/VEGFR2 y PTHrP/PTH1R, en la respuesta temprana a la estimulación mecánica para promover la viabilidad osteocítica.
Palabras clave: PTH1R, VEGFR2, estímulo mecánico, β-catenina, apoptosis.
Introduction
The skeleton adapts its mass, macro and micro architecture to changing mechanical forces [1]. Physical activity increases bone formation while immobilization increases bone resorption [2-4]. Osteocytes, mostly bone cells, are differentiated completed osteoblasts that are embedded in the mineralized matrix which form a mechanosensitive network. Transgenic mice with ablation of the osteocytes present trabecular and cortical bone loss [5]. This is consistent with the bone cells' ability to detect changes in mechanical loading and response, coordinating osteoblast and osteoclast function [6-8].
Accumulating evidence indicates that mechanical forces regulate the viability of osteocytes by ill-defined mechanisms. In vivo studies in rodents and in vitro in cultured osteocyte-like cells demonstrate that physiological levels of mechanical loading reduce osteocyte apoptosis, whereas the lack of mechanical stimuli promotes it [4,9,10]. Activation of the Wnt/β-catenin pathway, an important regulator of osteoblastic proliferation and differentiation [11], is essential for increased bone formation in response to mechanical loading [12,13]. Mechanical stimulation of osteocytes in the mouse ulna causes rapid activation of this pathway [13] associated with a reduced expression of Sost/sclerostin, an inhibitor of bone formation [14]. The role of prostaglandin E2 and NO has been suggested and the phosphatidylinositol 3 -kinase/Akt pathway in stabilizing β-catenin and cell survival by mechanical stimulation in osteocytes [15,16]. Recent findings indicate that mechanical stimulation promotes the formation of a signaling complex consisting of integrins, Src kinase, focal adhesion kinase and caveolin-1, resulting in the phosphorylation and nuclear translocation of extracellular signal-regulated kinase (ERK) [17,18].
The anabolic action of parathyroid hormone (PTH) has been shown to depend largely on its anti-apoptotic effect through the type 1 PTH receptor (PTH1R) in osteoblasts and osteocytes [19,20]. Mice heterozygous deletion of the gene for PTH-related protein (PTHrP), local counterpart bone, osteoblasts show osteopenia associated with decreased survival of osteoblasts and osteocytes [21]. Furthermore, mice with conditional suppression of PTH1R osteocytes specifically exhibit altered homeostasis of calcium and osteopenia [22]. In contrast, mice with constitutive overexpression of this receptor in the osteocytes show increased periosteal bone formation, associated with activation of the Wnt pathway and decreased osteoblastic apoptosis [23]. PTH1R's possible mediating role in the maintenance of bone mass by mechanical stimuli is, nevertheless, unknown. In this respect, there appears to be a synergistic effect of the mechanical load and the anabolic action of PTH on bone formation and resistance in the long bones of rats [24]. In vitro, the fluid flow has been shown to alter the conformation of PTH1R in osteoblastic cells MC3T3-E1 [25]. Moreover, also in vitro, mechanical stimulation induces gene expression of PTHrP local ligand of PTH1R in the bone, in osteoblastic cells and osteocytes [26]. Furthermore, the vascular endothelial growth factor (VEGF) is an important angiogenic factor, modulator of bone formation and repair, mainly through its receptor 2 (VEGFR2) [27]. VEGF / VEGFR2 system is an important mediator of proliferation, survival and differentiation of osteoblasts and osteoclasts [28,29]. The VEGFR2 mediates the actions of PTHrP on the differentiation and apoptosis in osteoblasts [29-31]. In endothelial cells, this receptor is activated by mechanical stimuli in a manner independent of ligand VEGF [32].
In the present study, we evaluated the possible involvement of PTHrP/PTH1R and VEGF/VEGFR2 systems in the survival of MLO-Y4 osteocyte cells promoted by mechanical stimulation.
Material and methods
Cell cultures: The MLO-Y4 and MLO-Y4-GFP cells, kindly provided by Dr. Lynda Bonewald (University of Missouri, Kansas City, Missouri, USA) and Dr. Teresita Bellido (Indiana University, Indianapolis, Indiana, USA), respectively were grown in culture medium α-MEM supplemented with fetal bovine serum (FBS) 2.5% calf serum (CS) 2.5% and 1% penicillin streptomycin in a humidified atmosphere of 5% CO2, at 37oC. Cells were cultured at a density of 20,000 cells / cm2 in culture dishes or glass slide both coated with collagen (FlexCell, Hillsborough, NC, USA); the next day fresh medium was added for 24 h. Then the cells were subjected or not (controls) to mechanical stimulation by shear stress or by laminar fluid flow or by several exposures to hypotonic medium, as described below. Cells were pre-incubated with PTHrP (1-36) (100 nM), generously supplied by Drs. A. F. Stewart and A. Garcia Ocana (Faculty of Medicine, University of Pittsburgh, Pennsylvania, USA.), or VEGF165 (6 ng / ml) (Calbiochem, Darmstadt, Germany)as agonists, or the following antagonists and inhibitors: [Asn [10], Leu [11], D-Trp [12]] PTHrP (7-34) amide [PTHrP (7-34)] (1 M) and JB 4250 (1 μM) [6]; VEGF neutralizing monoclonal antibody (0.1 mg/ml) (R & D Systems, Minneapolis, Minnesota, USA); or SU5416, an inhibitor of phosphorylation of VEGFR2 (1 M) (Calbiochem). These agents are added 30 min -1 hour prior to mechanical stimulation.
Mechanical stimuli: Cells were subjected or not (control) to fluid flow at a rate of 10 dynes/cm2, 8Hz, for 10 min in a Flexcell® Streamer® Shear [7] stress device. Osmotic shock was carried out by replacing the culture medium in the cell culture plate by a hypotonic solution (240 mOsm) for 1 h. Cell exposure to the isotonic solution (317 mOsm) was used as control. After mechanical stimulation, protein extracts were collected and the cells were incubated with pro-apoptotic agent (etoposide) for 6 h.
Immunocytochemistry: Cells were fixed with 2% p-formaldehyde and permeabilization treatment with 0.1% Triton in phosphate buffered saline (PBS). Non-specific binding was blocked with bovine serum albumin 5%, followed by overnight incubation with primary polyclonal anti-β-catenin rabbit (Abcam, Cambridge, Massachusetts, USA) in a cold, humid chamber. Cells were washed with 0.1% Triton-PBS before incubation for 1 h with anti-rabbit IgG conjugated with Alexa Fluor 546 (Invitrogen, Groningen, Netherlands). The micrographs were obtained using a fluorescence microscope.
Cell Transfection: Cells were transfected with a plasmid expressing a dominant negative VEGFR2 (dnVEGFR2), a plasmid overexpressed VEGFR2 (provided by Dr. Alex Ullrich, Max-Planck Institute of Biochemistry, Martinsried, Germany) or empty vector (pcDNA, Invitrogen) using Lipofectamine LTX Plus (Invitrogen) following the manufacturer's instructions.
Assays of cell death/apoptosis: The MLO-Y4 cells were exposed to etoposide (50 µM) for 6 h to induce apoptosis after mechanical stimuli. Cell viability was determined by trypan blue exclusion and apoptosis in MLO-Y4-GFP cells was assessed by visualizing chromatin condensation and / or nuclear fragmentation. The percentage of nonviable cells to total cell number was calculated in each case. Etoposide induced cell death in these cells represented 13.6±0.8% or 30.3±0.4%, by trypan blue exclusion or nuclear morphology respectively. These values were normalized to 100% in Figs. The corresponding values of untreated cells with etoposide were 1±0.5 and 1.2±0.5% respectively.
Western blotting: Analysis of sub-cellular fractionated samples (Pierce, Rockford, IL) was used to obtain extracts of membrane and nuclear protein. These extracts (25-30 g) were then separated by SDS-PAGE (8-12% polyacrylamide) and transferred to nitrocellulose membranes (GE-Amersham, Pittsburgh, Pennsylvania, USA). The membranes were blocked with 2.5% skimmed milk in 0.1% Tween-PBS at room temperature for 1 h and subsequently incubated overnight at 4C with the following rabbit polyclonal antibodies: anti-β-catenin (Abcam); anti-PTH1R (Ab-IV, Covance, Berkeley, California, USA); and anti-ERK1/2 (Cell Signaling, Beverly, Massachusetts, USA). As loading controls, the following antibodies were used: goat polyclonal anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, California, USA) or monoclonal mouse anti-α-tubulin (Santa Cruz Biotechnology). Then the corresponding secondary antibody coupled to horseradish peroxidase (Santa Cruz Biotechnology) was added. Detecting the luminescent signal in the membranes was performed with the ECL system (GE-Amersham) and band intensities were quantified using densitometry.
Statistical analysis: Results are expressed as mean ± SEM. Statistical analysis between two groups was performed using the Mann-Whitney. A p <0.05 was considered significant.
Results
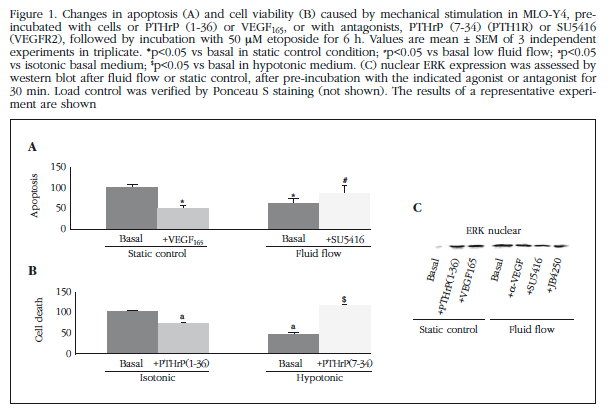
Treating MLO-Y4 osteocyte cells with two different methods of mechanical stimulation was found to protect etoposide-induced cells from death (Figure 1). Mechanical cell stimulation by fluid flow for 10 min at 10 dynes/cm2 protected from apoptosis induced by etoposide exposure for 6 h (Figure 1A). This protective effect was blocked by cell pretreatment with a selective inhibitor of VEGFR2, SU5416 (1 µM). Mechanical stimulus protection was reproduced by the pre-treatment of cells with 6 ng/ml of VEGF (Figure 1A). Furthermore, cells were submitted to mechanical stimulation by exposure to hypo-osmotic buffer for 1 h, which also induced protection against etoposide. This protective effect was blocked by pretreatment with PTH1R inhibitor, PTHrP (7-34) (Figure 1B). As observed previously with VEGF, PTHrP (1-36) pre-treatment reproduced the protective effect of osmotic shock (Figure 1B).
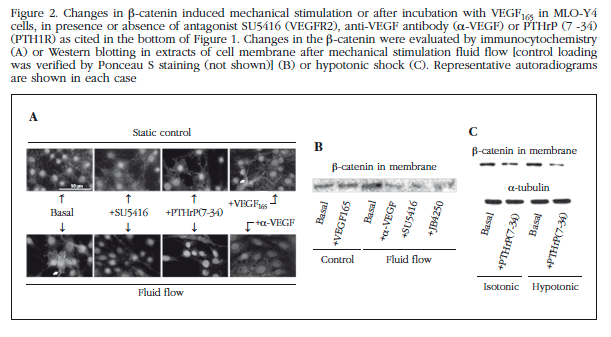
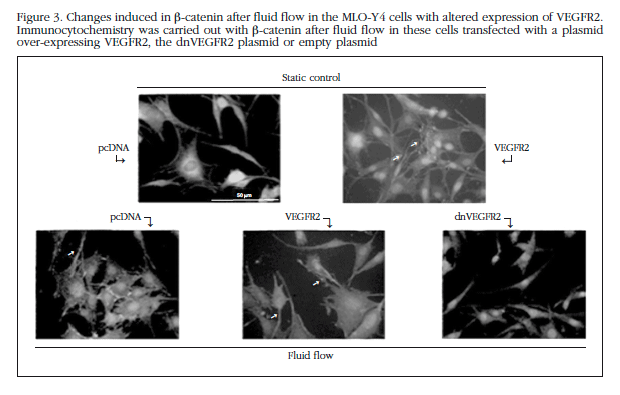
Translocation of ERK to the nucleus is a requirement for survival induced by mechanical stimuli. Thus, we observed that stimulation by fluid flow (10 min, 10 dynes/cm2) induced an increase in ERK in the nucleus of MLO-Y4 cells (Figure 1C). This effect was blocked by pretreatment with an anti-VEGF antibody, as well as inhibitors of VEGFR2 and PTH1R, SU5416 and JB4250, respectively (Figure 1C). It is also known that the Wnt/β-catenin pathway is involved in mechanotransduction in osteocytes. Observed by immunocytochemistry and Western that mechanical stimulation of MLO-Y4 cells induced rapid translocation of β-catenin to the cell membrane (Figures 2A and 2B) transfer; an effect blocked by antagonists VEGFR2 and PTH1R, SU5416 and PTHrP (7-34), respectively, and by an anti-VEGF antibody. Similarly, osmotic shock induced translocation of β-catenin membrane (Figure 1C). The involvement of VEGF/VEGFR2 system in mobilizing the β-catenin membrane by mechanical stimulation was also analyzed by transfection of MLO-Y4 cells with a plasmid that overexpresses VEGF or dnVEGFR2. Overexpression of VEGF in these cells reproduced translocation of β-catenin membrane; whereas this mobility induced by mechanical stimulation did not occur in cells with dnVEGFR2 (Figure 3).
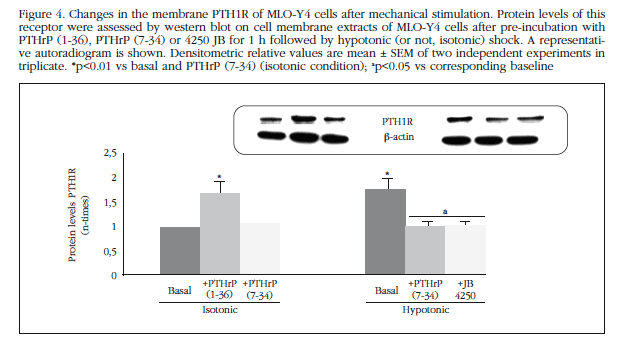
Furthermore, we wanted to study if mechanical stimulation modulated the location PTH1R in these membrane osteocytic cells. We note that both osmotic stress treatment and PTHrP (1-36) increased protein levels exogenous receptor in the membrane of MLO-Y4; while antagonists PTH1R, PTHrP (7-34) and JB 4250, blocked osmotic shock effects (Figure 4).
Discussion
Osteocyte viability, essential for the maintenance of bone mass and strength, is compromised in situations of osteopenia/osteoporosis [33,34]. Under physiological conditions, the viability of osteocytes remains critical levels of mechanical loading through poorly defined mechanisms [33]. In vitro studies in cells of MLO-Y4 have shown that stretching induces cellular anti-apoptotic response through a mechanism involving a complex signaling related to nuclear translocation ERK [4,18]. It has also been shown recently that the viability of MLO-Y4 cells induced by mechanical stimulation is modulated by the interaction between the pathways of caveolin-1/ERK and Wnt/β-catenin [18]. In this study we observed that both systems, PTHrP/PTH1R and VEGF/VEGFR2, are involved in protection against cell death by apoptosis which give the osteocytic cells two different mechanical stimuli, and osmotic shock fluid flow.
It has previously been shown to express the PTH1R osteocytes and respond to stimulation with PTH [35], an important calciotropa hormone responsible for calcium homeostasis in physiological conditions. Recent studies in genetically engineered mice indicate that the action of PTH requires a functional PTH1R in osteocitos [22]. From a pharmacological perspective, intermittent administration of PTH in mice attenuates rapidly osteoblast apoptosis in the vertebrae; this effect appears to be only a consequence of direct hormone action on osteoblasts, but also indirectly through its inhibitory effect on the expression of Sost/sclerostin in osteocytes [20,36,37]. Furthermore, in these cells PTH1R appears to play a key role in bone anabolic response to mechanical loading [38]. In this sense, described in rodents induced bone anabolism intermittent administration of PTH is enhanced by mechanical stimulation [25,39]. The functional interaction between mechanical stimulation and PTH is supported by in vitro studies using primary cultures of osteocytes [32]. Thus, the present data suggest that the osteocytes PTH1R integrates mechanical and hormonal for coordinated regulation of bone formation signals.
Moreover, our results indicate that VEGFR2 is critical for both the translocation of β-catenin to the cell membrane and for ERK to the nucleus. The system of VEGF is involved in the mechanisms of survival in various cell types including osteoblasts [29,30,40]. This growth factor promotes survival of endothelial cells by stimulating the formation of a multi-transmembrane protein complex that includes VEGFR2, VE-cadherin and β-catenina [40]. Our results demonstrate that, immediately after stimulation by fluid flow, the β-catenin was translocated to the membrane of MLO-Y4 osteocyte cells associated with VEGFR2 activation. The possibility that this may occur in vivo mechanism to explain the observed in response to mechanical stimulation requires further studies in animal models for osteocyte survival.
In summary, our in vitro results support an important role both for VEGFR2 and PTH1R as mechanisms that promote the viability of osteocytes after mechanical stimuli.
Conflict of interest
The authors declare no conflicts of interest.
![]() Correspondence:
Correspondence:
Pedro Esbrit
Laboratorio de Metabolismo Mineral y Óseo
IIS-Fundación Jiménez Díaz-UAM
Avda. Reyes Católicos, 2
28040 Madrid (España)
Correo electrónico:
pesbrit@fjd.es
Date of receipt: 15/09/2015
Date of acceptance: 06/11/2015
Bibliogragraphy
1. Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G, et al. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One 2013;8:e62172. [ Links ]
2. Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone 2002;30:781-6. [ Links ]
3. Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull 2003;16:45-54. [ Links ]
4. Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 2006;21:605-15. [ Links ]
5. Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 2007;5:464-75. [ Links ]
6. Rubin CT, Lanyon LE. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res 1987;5:300-10. [ Links ]
7. Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun 2006;348:1082-8. [ Links ]
8. You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 2008;42:172-9. [ Links ]
9. Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, et al. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 2003;284:C934-43. [ Links ]
10. Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Re Commun 2004;320:1163-8. [ Links ]
11. Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology 2007;148:2630-4. [ Links ]
12. Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 2006;281:23698-711. [ Links ]
13. Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 2006;281:31720-8. [ Links ]
14. Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866-75. [ Links ]
15. Kitase Y, Barragan L, Quing H, Kondoh S, Jiang JX, Johnson ML, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res 2010;25:2657-68. [ Links ]
16. Santos A, Bakker AD, Zandieh-Doulabi B, Blieck-Hogervorst JMA de, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun 2010;391:364-9. [ Links ]
17. Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol 2005;289:C633-43. [ Links ]
18. Gortázar AR, Martin-Millán M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/extracellular signal-regulated kinase (ERK) and beta-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem 2013;288:8168-75. [ Links ]
19. Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol 2013;85:1417-23. [ Links ]
20. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999;104:439-46. [ Links ]
21. Miao D, He B, Jiang Y, Kobayashi T, Sorocéanu MA, Zhao J, et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest 2005;115:2402-11. [ Links ]
22. Powell WF Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 2011;209:21-32. [ Links ]
23. O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 2008;3:e2942. [ Links ]
24. Ma Y, Jee WS, Yuan Z, Wei W, Chen H, Pun S, et al. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibialdiaphyseal cortical bone. J Bone Miner Res 1999;14:439-48. [ Links ]
25. Zhang YL, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am J Physiol Cell Physiol 2009;296:C1391-9. [ Links ]
26. Chen X, Macica CM, Ng KW, Broadus AE. Stretch-induced PTH-related protein gene expression in osteoblasts.J Bone Miner Res 2005;20:1454-61. [ Links ]
27. Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Löwik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000;141:1667-74. [ Links ]
28. Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, et al. Skeletal defects in VEGF (120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 2002;129:1893-904. [ Links ]
29. Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 2002;111:61-73. [ Links ]
30. Alonso V, Gortázar AR, Ardura JA, Andrade-Zapata I, Alvarez-Arroyo MV, Esbrit P. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol 2008;217:717-27. [ Links ]
31. Gortázar AR, Alonso V, Alvarez-Arroyo MV, Esbrit P. Transient exposure to PTHrP (107-139) exerts anabolic effects through vascular endothelial growth factor receptor 2 in human osteoblastic cells in vitro. Calcif Tissue Int 2006;79:360-9. [ Links ]
32. Jin ZG, Ueba H, TanimotoT, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 2003;93:354-63. [ Links ]
33. Boyce BF, Xing L, Jilka RL, Bellido T, Weinstein RS, Parfitt AM, et al. Principles of Bone Biology. Bilezikian JP, Raisz LG, Rodan GA (eds.). San Diego, CA: Academic Press 2002;151-68. [ Links ]
34. Manolagas. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endoc Rev 2000;21:115-37. [ Links ]
35. Bringhurst FR. PTH receptors and apoptosis in osteocytes. J Musculoskel Neuronal Interact 2002;2:245-51. [ Links ]
36. Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 2004;35:828-35. [ Links ]
37. Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 2005;146:4577-83. [ Links ]
38. Tu X, Pellegrini G, Galli C, Benson JD, Condon KW, Bivi N, et al. PTH receptor 1 expression in osteocytes is indispensable for the anabolic effect of mechanical loading in mice. J Bone Miner Res 2011;25:S24. [ Links ]
39. Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone 2008;43:238-48. [ Links ]
40. Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008;121:2115-22. [ Links ]











 texto en
texto en