Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 no.2 Madrid abr./jun. 2016
Comparison between two automated chemiluminescence immunoassays for quantifying 25 (OH) vitamin D
Comparación entre dos inmunoensayos automatizados por quimioluminiscencia para la cuantificación de 25(OH) vitamina D
Torrubia B.1, Alonso I.1, López-Ramiro E.1, Mahillo I.2 and De la Piedra C.1
1 Laboratorio de Bioquímica
2 Servicio de Epidemiología.
Fundación Jiménez Díaz - Madrid (España)
Work submitted as FEIOMM benefit the grant received to attend the 34th Congress of the ASBMR (Minneapolis, 2013).
This study was funded by the Laboratorios Fujeribio Europe.
SUMMARY
Introduction: Quantifying total blood 25 (OH) vitamin D is the most accurate marker of an individual's vitamin D status. The gold standard technique for measurement is liquid chromatography tandem mass spectrometry (LC-MS/MS), although currently clinical laboratories tend to use chemiluminescence techniques. The objective of this study was to compare 25 (OH) vitamin D concentrations obtained by two commercially-produced automated methods and study the correlation of these methods with the LC-MS/MS reference technique.
Material and methods: The 25(OH) vitamin D levels were quantified in 1,000 serum samples from theJimenez Diaz Biochemistry Foundation Laboratory using 2 automated methods for chemiluminescence detection:ADVIA CENTAURO® (SIEMENS) and LUMIPULSE® G1200 (Fujirebio). Among all the samples tested, the 50 most discordant to each other were sent to be evaluated by LC-MS/MS reference technique.
Results: The results indicate that there is good correlation between the two methods: CCI=0.923 (0.914-0.932), with the G1200 LUMIPULSE® values 10% being higher than CENTAURO®. Regarding the 50 samples selected, we can see that there is a good correlation between the two immunoassays with LC-MS/MS, although both methods significantly underestimate 25 (OH) vitamin D results with respect to the gold standard.
Discussion: Although both techniques are suitable for use, it is worth considering whether the worldwide vitamin D deficiency epidemic is due to the analysis methodology used. This variability between immunoassays could be solved by standardizing the different commercial techniques in line with NIST-produced reference materials.
Key words: 25(OH) vitamin D, Fujirebio, SIEMENS, technical comparison, LC-MS/MS.
RESUMEN
Introducción: La cuantificación de 25(OH) vitamina D total en sangre es el marcador más preciso del estado de vitamina D en un individuo. La técnica patrón-oro para su medición es la cromatografía líquida/espectrometría de masas en tándem (LC-MS/MS), aunque actualmente los laboratorios clínicos utilizan de rutina técnicas de quimioluminiscencia. El objetivo del estudio fue comparar las concentraciones de 25(OH) vitamina D obtenidas mediante dos métodos automatizados comerciales y estudiar la correlación de dichos métodos con la técnica de referencia LC-MS/MS.
Material y método: Se cuantificaron los niveles de 25(OH) vitamina D en 1.000 muestras de suero del laboratorio de Bioquímica de la Fundación Jiménez Díaz mediante 2 métodos automatizados comerciales por detección de quimioluminiscencia: ADVIA CENTAURO® (SIEMENS) y LUMIPULSE® G1200 (FUJIREBIO). Entre todas las muestras analizadas, las 50 más discordantes entre sí se enviaron para ser evaluadas por la técnica de referencia LC-MS/MS.
Resultados: Los resultados obtenidos indican que existe una buena correlación entre los dos métodos: CCI=0,923 (0,914-0,932), siendo los valores de LUMIPULSE® G1200 un 10% superiores a los de CENTAURO®. Con respecto a las 50 muestras seleccionadas, podemos observar que existe una buena correlación entre los dos inmunoensayos con la técnica LC-MS/MS, aunque ambos métodos infraestiman considerablemente los resultados de 25(OH) vitamina D con respecto al patrón-oro.
Discusión: Aunque ambas técnicas son adecuadas para su utilización, habría que plantearse si la "epidemia" mundial de hipovitaminosis D se debe a la metodología de análisis utilizada. Esta variabilidad entre inmunoensayos se solucionaría estandarizando las diferentes técnicas comerciales con los materiales de referencia elaborados por el NIST.
Palabras clave: 25(OH) vitamina D, FUJIREBIO, SIEMENS, comparación técnica, LC-MS/MS.
Introduction
Vitamin D is a fat-soluble vitamin involved in calcium and phosphorus metabolism whose role is essential in bone formation and mineralization. Currently, its immunomodulatory actions, antiproliferative and cell differentiation stimulatory of associated pathologies such as cardiovascular diseases, diabetes and cancer have also been shown [1,2].
Quantification of 25 (OH) vitamin D total blood is the most accurate marker of vitamin D status in an individual, although its active metabolite is 1,25 (OH)2 vitamin D [3,4].
The gold standard technique for measurement is liquid chromatography/tandem mass spectrometry (LC-MS/MS), but clinical laboratories routinely used chemiluminiscence immunoassays [5].
The main problems of these immunoassays are due to the hydrophobic nature of the analyte, the high concentration in which vitamin D binding protein (DBP) in serum is found, and the existence of cross reactions of multiple vitamin D with the metabolite antibodies used in the process. Therefore, proper immunoassay requires a pretreatment to inactivate DBP, a careful selection of antibody used and the standardization of the technique compared to the values returned by LC-MS/MS analyzer. Different commercial techniques for analysis of vitamin D differ in how to separate the binding protein, the percentage of crossed reactions with other metabolites of our analyte, as well as the specificity of the antibody used [4,6].
Currently, as a result of general knowledge on severe vitamin D deficiency in the world's population, it has become necessary to measure vitamin D levels in different populations, research cohorts and individual patient [7]. Several studies have shown considerable variation between the different analytical methods based on immunochemistry, liquid/UV and LC-MS/MS chromatography. It has been argued that a particular patient can be classified with levels of sufficiency or insufficiency of vitamin D depending on the laboratory where this analysis is carried out [7,8].
To solve this problem, the need for standardization of the levels of 25 (OH) vitamin D has been established by numerous scientific organizations. In 2011, the Office of Dietary Supplements (ODS) of the National Institutes of Health US (NIH) in collaboration with the National Institute of Standards and Technology (NIST) created the program standardization of vitamin D (VDSP). NIST has developed 4 based reference materials with different serum concentrations of vitamin D known order to standardize the various commercial techniques [7,9]. However, not all the current methods used to quantify 25(OH) vitamin D already calibrated against these accepted standards [10].
The aim of this study was to compare 25(OH) vitamin D concentrations obtained using two commercial automated methods and study the correlation of these methods with the LC-MS/MS technique.
Material and methods
We used 1,000 serum samples selected at random from those tested in FJD's clinical analysis laboratory. The samples were obtained from patients aged between 1 and 92 years (59±18, average ± SD) with 37% of women and 63% men. Levels of 25 (OH) vitamin D were quantified with ADVIA Centaur XP® (SIEMENS) and LUMIPULSE® G1200 (FUJIREBIO).
In all cases the samples were handled anonymously, so obtaining informed consent of patients was not required.
The analyzer LUMIPULSE® G1200 (FUJIREBIO) takes a noncompetitive sandwich-type immunoassay with chemiluminescent detection using two antibodies, a monoclonal sheep antibody that binds to 25(OH) vitamin D2 and D3, and a second monoclonal antibody that binds exclusively to the complex formed above. Separation of vitamin D binding protein is carried out by a chemical agent in 1st reaction.
According to the manufacturer's specifications, the technique has an intra-assay imprecision with a coefficient of variation (CV) <6% and a functional sensitivity of 3.491 ng/mL. Measurement interval is 4-150 ng/mL. Analytical specificity reflected by the percentage of cross-reactivity with other metabolites is 100% for 25(OH) vitamin D3, 100.1% for 25(OH) vitamin D2, and 19.9% for the epimer C3 25(OH) vitamin D3.
The ADVIA Centaur XP® (SIEMENS) is a competitive immunoassay with chemiluminescent detection using an anti-fluorescein murine monoclonal antibody covalently coupled to paramagnetic particles (PMP), a murine monoclonal anti-25(OH) vitamin D marked with acridinium ester, and vitamin D analog with fluorescein. As separation medium binding protein release agent is used in buffered saline.
According to the manufacturer's specifications, the technique presents an intra-assay imprecision with a CV of 4.2% -11.9% and a functional sensitivity of 4.2 ng/mL. Measurement interval is 4.2 to 150 ng/mL. Analytical specificity reflected by the percentage of cross-reactivity with other metabolites is 97.4% for the 25(OH) vitamin D3, from 106.2% for 25 (OH) vitamin D2 and 1% for C3 epimer 25(OH) vitamin D3.
Among all samples tested, the 50 discordant with each other were sent to be evaluated by LC-MS/MS method in the laboratory of Dr. Etienne Cavalier (Department of Clinical Chemistry, University of Liege, Belgium); in order to compare the two chemiluminescent immunoassays relative to the reference technique LC-MS/MS. The difference between these results 50 samples ranged between 14% and 133% (32±52%, mean ± SD) with respect to the average of the two values obtained. In all cases the percentage was higher than the coefficients of variation inter-analysis of the two methods: FUJIREBIO, 6%; SIEMENS, 11.9%. We analyzed the most discordant samples to ascertain whether they belonged to a particular group of patients. For example, pregnant women who have abnormal levels of vitamin D binding protein. However, we note that these patients belonged mainly to Nephrology, Rheumatology and Endocrinology, an insignificant fact since these services are most demanded the determination of vitamin D. Moreover, the average age of these 50 patients was 63±16 years, with 34% male and 66% female, figures which were quite similar to the total group of 1,000 samples (age 59±18 years with 37% of women and 63% men). The most discordant samples were chosen together to analyze for gas-mass because our goal was twofold. First, to check its similarity to the technique of gas-mass and otherwise clarify which of the two techniques was closer to the reference technique. This second point could not clarify if we sent the samples whose results were similar. Regarding the choice of 50 samples as appropriate for the study was done because it was a sufficient amount to obtain statistically significant results samples. Due to the high cost of gas-mass determination it was not possible to send a larger number of samples.
Results
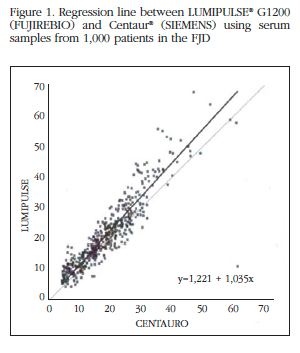
We assessed the degree of concordance of vitamin D measures provided by the two appliances: ADVIA Centaur XP® and LUMIPULSE G1200®. To do this, we calculated the intraclass correlation coefficients (ICC) with confidence intervals at 95%. The results indicate a good correlation between the two methods: CCI=0.923 (0.914 to 0.932). There are no significant differences in the ICC if the samples are divided into groups with vitamin D levels <20 ng/mL and >20 ng/mL.
The regression line obtained from both trials was Y=1.221+1,035X, where Y corresponds to the values of LUMIPULSE G1200 and X to Centaur XP®. LUMIPULSE G1200 values were 10% higher than Centaur® (Figure 1).
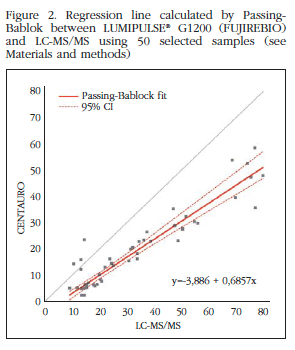
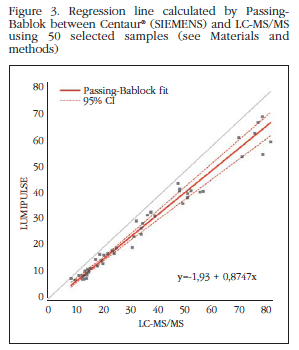
With respect to 50 samples selected subgroup to analyze by LC-MS/MS was obtained with ICC=0.987 LUMIPULSE and CCI=0.938 with Centaur®. Although both are satisfactory, the intraclass correlation coefficient is the highest LUMIPULSE therefore measurements of this device is more like the exact ones (Figures 2 and 3).
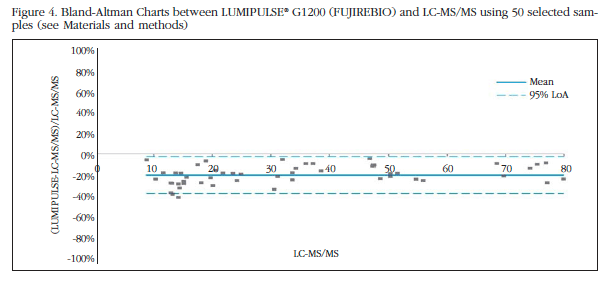
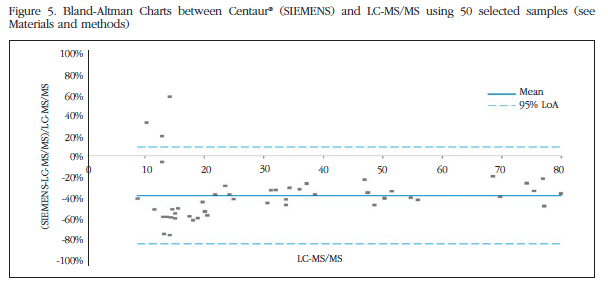
Then with our subgroup of 50 selected samples, we carried out the Bland-Altman plots, where the X axis corresponds to the mean of each pair of observations and the Y axis differences between each pair of observations. In the graphs there are two horizontal solid lines. The gray solid line is drawn at zero height; if the measurements given by the apparatus were identical to the exact measurement points should be located right on this line. The solid blue line represents the mean of the differences. If this line is the line below the 0 value means that the device tends to lower measures the exact value, and if it is above the opposite.
As can be seen in figure 4, the mean difference between the analyzer LUMIPULSE® G1200 and the reference method LC-MS/MS is 20%; therefore this immunoassay underestimates values of 25 (OH) vitamin D by 20% compared to the gold standard. In figure 5, we see how in the case of the Centaur® the average of the differences is 42%, so the values which casts this technique are much lower than those of the reference method. Furthermore, the technique LUMIPULSE® G1200 is less spread in the results.
Discussion
Both methods have a good correlation between them, the values obtained in the Centaur® approximately 10% lower than those obtained by the LUMIPULSE® G1200.
The correlation of both immunoassays with the reference technique LC-MS/MS is good (although higher for LUMIPULSE that Centaur), which does not exclude that the two methods considerably underestimate the results of 25(OH) vitamin D with respect to gold standard. In this selection of samples, held by choosing those where the discrepancy between the two methods was higher, LUMIPULSE® G1200 underestimates the values by 20%, while yields Centaur® 25 (OH) vitamin D 42% lower than the reference method LC-MS/MS. This difference between the two immunoassays is given by the different technique used (competitive assay in SIEMENS and noncompetitive sandwich in FUJIREBIO), pretreatment of the sample to separate the 25 (OH) vitamin D and DBP selected antibodies.
Currently, according to studies, more than half of the world's population has insufficient levels or even frank deficiency of vitamin D [11]. This may be considered a global "epidemic", but should be questioned as to whether this state of widespread vitamin deficiency is largely influenced by the analysis methodology used in determining concentrations of 25(OH) vitamin D [8,11].
This variability between immunoassays would be solved by standardizing different commercial techniques with reference materials for measuring 25(OH) vitamin D produced by the NIST [12].
Acknowledgements
Special thanks to Alicia Nadal for her revisions and collaboration throughout the development of this study.
Competing interests
The authors declare no conflict of interest.
![]() Correspondence:
Correspondence:
Concha de la Piedra
Laboratorio de Bioquímica
Fundación Jiménez Díaz
Avda. Reyes Católicos, 2
28040 Madrid (España)
e-mail: cpiedra@fjd.es
Date of receipt: 23/12/2015
Date of acceptance: 16/03/2016
Bibliography
1. Rojas-Rivera J, De La Piedra C, Ramos A, Ortiz A, Egido E. The expanding spectrum of biological actions of vitamin D. Nephrol Dial Transplant. 2010;25(9):2850-65. [ Links ]
2. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81. [ Links ]
3. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30. [ Links ]
4. Ofenloch-Haehnle B. Approaches to measurement of vitamin D concentrations - immunoassays. Scand J Clin Lab Invest Suppl. 2012;243:50-3. [ Links ]
5. Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441-8. [ Links ]
6. Kobold U. Approaches to measurement of vitamin D concentrations - mass spectrometry. Scand J Clin Lab Invest Suppl. 2012;243:54-9. [ Links ]
7. Thienpont LM, Stepman HC, Vesper HW. Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest Suppl. 2012;243:41-9. [ Links ]
8. Ibrahim F, Parmentier C, Boudou P. Divergence in classification of 25-hydroxyvitamin D status with respect to immunoassays. Clin Chem. 2007;53(2):363-4. [ Links ]
9. Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88:511S-2S. [ Links ]
10. Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75(7):477-88. [ Links ]
11. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006,81:353-73. [ Links ]
12. Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82:1942-8. [ Links ]











 texto en
texto en