Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.9 no.4 Madrid nov./dic. 2017
https://dx.doi.org/10.4321/s1889-836x2017000400006
Originals
The effect of oxidative stress on vascular calcification through microRNA-377
1Servicio de Metabolismo Óseo y Mineral - Instituto de Investigación Sanitaria del Principado de Asturias - Red de Investigación Renal (REDinREN) del Instituto de Salud Carlos III (ISCIII) - Universidad de Oviedo - Hospital Universitario Central de Asturias - Oviedo (España)
Introduction
Vascular calcification (VC) is one of the most common disorders in aging, but especially in patients with chronic kidney disease (CKD). The increase of reactive oxygen species (ROS) in response to overloads in phosphorus (P) which occurs during VC contributes to the phenotypic dedifferentiation of vascular smooth muscle cells (VSMC) from a properly muscular (contractile) phenotype to an osteoblastic phenotype 1,2,3,4. In particular, hydrogen peroxide is considered one of the most common ROS, capable of inducing the expression of the osteoblastic transcription factor Cbfa1/RUNX2 and inducing calcification 5,6.
MicroRNAs (miRs) are small, non-coding single stranded RNAs (~22 nucleotides) that mediate the posttranscriptional silencing of genes by binding through complementarity of bases to the 3 'UTR region of the target mRNAs. The miRs are involved in crucial biological processes, including cell proliferation, differentiation and tissue development 7,8. Recent studies have shown that several miRs are important regulators of VSMC differentiation into cells resembling osteoblasts and, therefore, vascular calcification 9,10,11,12.
The miR-377 appears as an important aging regulator by regulating, among others, superoxide dismutase 2 enzyme (SOD-2) 13,14. SOD is the body's main antioxidant, which catalyzes the conversion of superoxide radicals to hydrogen peroxide. SOD-2 corresponds to the mitochondrial form of the enzyme. Several articles have considered the role of oxidative stress as an inducer of VC, so in the present study we propose to analyze the possible role of miR-377 as regulator of aortic mineralization in an in vivo model.
Material and methods
Model of vascular calcification
Our research protocol was approved by Oviedo University’s Ethics Committee for Animal Experimentation. The study was carried out using male Wistar rats (n=10) at 4 months of age (350-400 g) who underwent chronic renal failure (CRF) (7/8) in a single surgical procedure, after isoflurane inhalation anesthesia. Complete nephrectomy of the right kidney and then subtotal left kidney nephrectomy were carried out by lateral incision and in the upper area. With this procedure, approximately one-quarter of the renal mass is conserved. CRF rats were divided into two groups: one, NP, fed a standard rodent diet with a normal P content (0.6%) and 0.6% calcium (Ca), and a protein content of 20% (Panlab, Barcelona, Spain); The other, HP group, was fed a diet high in P (0.9%), 0.6% Ca, and a protein content of 20% (Panlab, Barcelona, Spain). The study lasted 20 weeks in order to induce vascular calcifications. A Sham group (n=5) was also included and followed up to week 20. Twenty-four hours before slaughter, the rats were housed in metabolic cages to obtain urine samples in each case receiving the same diet and water ad libitum. They were sacrificed using CO2 anesthesia, and serum samples were taken for analysis. The descending abdominal aorta was removed to the iliac bifurcation from each rat and divided into three portions: the first fragment closest to the aortic arch to determine the Ca content, the second fragment was used for RNA extraction and proteins, and the third fragment was included in paraffin for future studies.
Two tibias were removed at the time of sacrifice. The left was preserved in alcohol to measure bone mineral density (BMD). The remaining tibia was frozen at -80°C.
Biochemical markers
Serum Ca, P and creatinine and urine creatinine levels were measured using a Hitachi 717 multi-channel automatic analyzer (Boehringer Mannheim, Berlin, Germany). Serum parathormone (PTH) was measured by ELISA (Immutopics, San Juan Capristano, USA) following the manufacturer's protocols. Fibroblast growth factor 23 (FGF23) was determined by an ELISA kit (Kainos Laboratories, Japan).
Bone densitometry
BMD was measured in the tibia at three levels: proximal octave, seventh/eighth distal and total tibia, with a Hologic QDR-1000 dual-energy digital radiological densitometer (Hologic, Bedford, USA) equipped with a specific program for small animals.
Analysis of aortic calcification
Abdominal aortic calcification of the rats was analyzed for total Ca content. To determine this, a fragment of the abdominal aorta (closest to the aortic arch) was homogenized with an Ultra-Turrax (OmniHT) in 0.6 N HCl. After stirring at 4°C for 24 hours the samples were centrifuged. Ca content was determined in the supernatant by the o-cresolphthalein complexone method (Sigma-Aldrich, St. Louis, USA), and the cell pellet was resuspended in lysis buffer (125 mM Tris and 2% SDS, PH 6.8) for protein extraction and quantification by the Lowry assay (Bio-Rad, Hercules, USA). The Ca content was normalized by expressing as µg Ca per mg of protein.
Study of gene expression
RNA extraction was carried out by the guanidinium-phenol-chloroform thiocyanate method. The DNA copy (cDNA) was synthesized using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, USA). Gene expression of miR-377 was analyzed by real-time PCR (qPCR) in the Stratagene Mx3005P QPCR System (Agilent Technologies, Santa Clara, USA). An assay on-demand designed by Applied Biosystems employing specific oligos and fluorescent TaqMan probes was used for the PCR. For quantification and normalization, nuclear RNA U6 expression was used.
Western Blot
After transfer, the membranes were incubated for 12 hours with anti-SOD-2 (1:1000, Cell Signaling Technology, Danvers, USA) and anti-GAPDH (1:5000, Santa Cruz Biotechnology, Dallas, USA.). Binding of the secondary antibody was detected with the Western Blot detection kit ECL Advance (Amersham Bioscience, Buckinghamshire, UK) and the VersaDoc 4000 imaging system (Bio-Rad).
Statistic analysis
The SPSS 17.0 program was used for the statistical analysis of the results. In the case of variables with normal distribution, the comparison of the treatment groups was carried out using the analysis of variance (ANOVA) with the Bonferroni test. In the case of variables with non-normal distribution, the Kruskal-Wallis test was used.
Results
Nephrectomy decreased creatinine clearance, an effect that did not worsen in animals fed the high P diet (Table 1). There was a discrete but not significant increase in serum P in the nephrectomized animals, which became more noticeable in those who received the high P diet. However, small increases in serum P were associated with more significant increases in serum levels of FGF23. Serum FGF23 levels increased twice with nephrectomy, while serum P increased by less than 50%. Similarly, in animals that received the diet high in P (CRF+HP), FGF23 levels increased twice as much as those in the normal diet (CRF+NP), with an increase in serum P less than 25%.
Table 1 Biochemical and bone metabolism markers in the different treatment groups. BMD values in the three tibial segments analyzed in the different treatment groups

ap<0.05 relative to the Sham group; bp<0.05 relative to the CRF+NP group.
Nephrectomy had no effect on BMD changes in tibia, although CRF+HP in BMD in proximal tibia decreased significantly with respect to the CRF+NP group and the Sham group (Table 1).
In the CRF+NP group, Ca content in the aorta was increased 3-fold with respect to the Sham group. The increase with respect to the Sham group was increased up to 17 times in the CRF+HP group. The miR-377 expression decreased by 65% with nephrectomy, with no additional effect observed with the high-P diet. In fact, creatinine clearance was the biochemical parameter that was most strongly associated with the expression of this miR (R=0.83, p=0.001). In contrast, serum P was not associated with miR-377 expression (r=-0.302; p=0.34).
Protein expression of SOD-2 was clearly increased with nephrectomy (more than 3-fold), an effect that became more marked in nephrectomized animals with the high-P diet (more than 6-fold).

Figure 1 Ca content in aortas of 7/8 nephrectomy rats fed a diet with normal P content (0.6%) (CRD+NP) and high P content (0.9%) (CRD+HP), sacrificed at 20 weeks. Data represent the mean ± standard deviation. ap<0.05 relative to the Sham group; bp<0.05 relative to the CRD+NP group
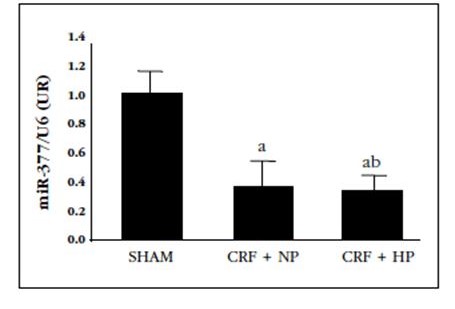
Figure 2 Relative levels of miR-377 in aortas of 7/8 nephrectomy rats fed a diet with normal content in P (0.6%) (CRF+NP) and high P content (0.9%) (CRD+HP), sacrificed at 20 weeks. Data represent the mean ± standard deviation. ap<0.05 relative to the Sham group
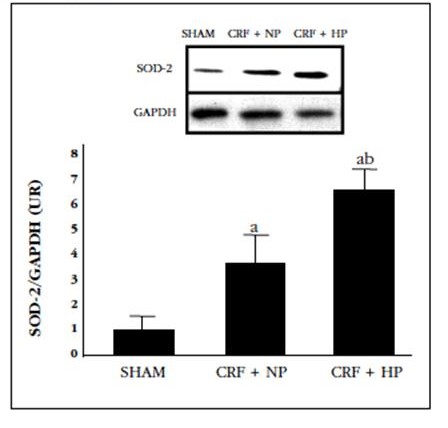
Figure 3 Protein expression of SOD-2 in the aortas of 7/8 nephrectomy rats fed a diet with normal content in P (0.6%) (CRF+NP) and high P content (0.9%) (CRD+HP), sacrificed at 20 weeks. A) Image of a Western blot representing one aorta of each group; B) graphical representation of the protein expression of SOD-2 in the different groups as mean ± standard deviation. ap 0.05 compared to the Sham group; bp<0.05 compared to the CRD+NP
Discussion
In this study, a decrease in aortic expression of miR-377 with nephrectomy was observed in parallel with the increase in SOD-2 protein expression as a possible compensatory mechanism to eliminate superoxide free radicals produced as a consequence of oxidative stress generated by nephrectomy.
To date, several hundred miRs have been identified in the human genome by proposing that at least 50% of the genes coding for proteins are regulated by miRs 15,16.
The increase in the protein expression of SOD-2, as a possible compensatory effect to curb oxidative stress and cellular apoptosis in the mitochondria 4,5,17, has been observed in our group’s other in vitro studies exposing VSMC to calcifying media. In fact, in a recent article we observed using two-dimensional gel-free proteomics techniques that, in the presence of a calcifying stimulus with high doses of calcitriol, the expression of this protein increases 3 fold with respect to the cells cultured with a control medium 18. Another in vitro study in VSMC subjected to a calcification stimulus by excess Ca and P shows an increase in the protein expression of SOD-2 by Western blot 6.
However, unlike what we have reported in our work, most studies in the literature show SOD-2 decreases in the presence of increased oxidative stress. In fact, in a recent study in rats with CRF, it has been found that the administration of a high P diet supplemented with calcitriol induces a decrease in the protein expression of SOD-2 at the aortic level 19. Other studies in other tissue models (renal podocytes and mesangial cells) have found that, in the case of stimuli inducing oxidative stress, there is a decrease in SOD-2 with increases in miR-377 (20). These increases have also been implicated with the increase of cellular senescence 14,21.
SOD-2 is also referred to as mitochondrial superoxide dismutase or manganese superoxide dismutase (MnSOD). It is responsible for the reduction of ROS toxic to mitochondria.
The fact that in our case an increase in SOD-2 is observed, in the cell’s attempt to defend itself against oxidative stress, accompanied by a decrease in miR-377 expression, may be attributed to different experimental conditions from the rest of the studies, as well as the time frame of renal damage, which in our case is always very long term. What does concur with all published reports is the opposite effect between miR-377 and SOD-2 14,20,21. That is, increases in SOD-2 lead to decreases in miR-377 expression and vice versa, so the possible usefulness of miR-377 as a biomarker of oxidative stress and vascular damage may be inferred.
Despite using a high P diet, serum P levels were very similar in the nephrectomized animal groups with normal or high P diet. One possible explanation is that 7/8 nephrectomy has not been aggressive enough so a lower grade of nephrectomy may be 5/6 or less. This would indicate maintaining a residual renal function that would avoid aggravating the renal damage caused by P 22. However, FGF23 notably increases with nephrectomy and also in animals that received the high-P diet. FGF23 is known to be the first biochemical parameter to rise after CRF 23. FGF23 begins to increase in the plasma of patients with CRD from very early situations, and continues to increase steadily as glomerular filtration declines 24. During the early stages of CRD, an overload of P would stimulate the synthesis of FGF23 at the osteocyte level, and would act on the remaining nephrons by increasing the fractional excretion of phosphate to maintain normal phosphatemia 25. In fact, in our case, increases of less than 25% in serum P were accompanied by much higher FGF23 variations as a mechanism to compensate for the increase in P in order to curb their excess.
The fact that kidney dysfunction obtained by nephrectomy was lower than initially predicted is reflected in the BMD results in the tibia. There were no differences in BMD between Sham animals and nephrectomized animals, despite the time course of CRD (20 weeks) 26. In the nephrectomized animals that received the high P diet, there was a decrease in BMD in proximal tibia compared to the nephrectomized animals with normal diet in P and in the Sham group. However, other studies in our group, with the same follow-up time, show a more negative effect on cortical bone (BMD in distal tibia) than on the trabecular (BMD in proximal tibia) when the diet administered is rich in P 27. This effect is accompanied by severe secondary hyperparathyroidism that mainly affects the cortical bone, a result that we did not observe in the present study.
Despite the lower degree of renal dysfunction observed, we detected a relationship between the VC increase and the decrease in BMD, as previously described by our group 28. The group of animals with CRF and high P diet showed a clear increase in calcium content in the aorta, which was accompanied by the higher losses of BMD in the proximal tibia.
From the results of this study, nephrectomy, independent of serum P levels, is capable of modifying miR-377 expression. The high P content in the diet induced increased protein expression of SOD-2, probably as a protective mechanism to avoid further vascular damage due to oxidative stress. These results alert us to the desirability of maintaining serum levels of P when renal deterioration is accentuated thus avoiding progressive vascular damage. The use of miR-377 as a biomarker of vascular damage due to oxidative stress requires mechanistic studies, but opens a possible preventive and therapeutic route to the development and progression of vascular lesions in CRD.
REFERENCES
1. Xie C, Ritchie RP, Huang H, Zhang J, Chen YE. Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol. 2011;31:1485-94. [ Links ]
2. House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769-85. [ Links ]
3. Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol. 2013;9:661-70. [ Links ]
4. Sutra T, Morena M, Bargnoux AS, Caporiccio B, Canaud B, Cristol JP. Superoxide production: a procalcifying cell signalling event in osteoblastic differentiation of vascular smooth muscle cells exposed to calcification media. Free Radic Res. 2008;42:789-97. [ Links ]
5. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319-27. [ Links ]
6. Roman-Garcia P, Barrio-Vazquez S, Fernandez-Martin JL, Ruiz-Torres MP, Cannata-Andia JB. Natural antioxidants and vascular calcification: a possible benefit. J Nephrol. 2011;24:669-72. [ Links ]
7. Bartel PD. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-97. [ Links ]
8. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93-103. [ Links ]
9. Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, et al. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc. 2012;1:e003905. [ Links ]
10. Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344-52. [ Links ]
11. Rangrez AY, M'Baya-Moutoula E, Metzinger-Le Meuth V, Hénaut L, Djelouat MS, Benchitrit J, et al. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement of miR-223. PLoS One. 2012;7:e47807. [ Links ]
12. Panizo S, Naves-Díaz M, Carrillo-López N, Martínez-Arias L, Fernández-Martín JL, Ruiz-Torres MP, et al. MicroRNAs 29b, 133b, and 211 regulate vascular smooth muscle calcification mediated by high phosphorus. J Am Soc Nephrol. 2016;27:824-34. [ Links ]
13. Duan S, Wang Y, Wang H, Wang S, Ji L, Dai D, et al. A novel PCR-based approach to discover miRNA target genes. Int J Med Sci. 2014;11:1270-4. [ Links ]
14. Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22:4126-35. [ Links ]
15. Jackson, RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE. 2007;367re1. [ Links ]
16. Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23-9. [ Links ]
17. Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002; 277: 33242-8. [ Links ]
18. Carrillo López N, Tuñón LePoultel D, Quirós Caso C, Rodríguez I, Cannata Andía JB, Naves Díaz M. Efecto de dosis suprafisiológicas de calcitriol sobre la expresión proteica de células de músculo liso vascular. Rev Osteoporos Metab Miner. 2017 (Epub ahead of print). [ Links ]
19. Agharazii M, St-Louis R, Gautier-Bastien A, Ung RV, Mokas S, Larivière R, et al. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens. 2015;28:746-55. [ Links ]
20. Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue QC, et al. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med. 2015;83:214-26. [ Links ]
21. Xie HF, Liu YZ, Du R, Wang B, Chen MT, Zhang YY, et al. miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1. Cell Death Dis. 2017;8(3):e2663. [ Links ]
22. Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22: 2909-16. [ Links ]
23. Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975-80. [ Links ]
24. Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305-15. [ Links ]
25. Lloret MJ, Bover J, DaSilva I, Furlano M, Ruíz-García C, Ayasreh N, et al. Papel del fósforo en la enfermedad renal crónica. Nefrología (Suplemento Extraordinario). 2013;4:2-10. [ Links ]
26. Naves-Díaz M, Carrillo-López N, Rodríguez-Rodríguez A, Braga S, Fernández-Coto T, Lopez-Novoa JM, et al. Differential effects of 17A-estradiol and raloxifene on bone and lipid metabolism in rats with chronic kidney disease and estrogen insufficiency. Bone. 2010;17:766-71. [ Links ]
27. Carrillo-Lopez N, Panizo S, Alonso-Montes C, Román-García P, Rodríguez I, Martínez-Salgado C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90:77-89. [ Links ]
28. Román-García P, Carrillo-López N, Fernández-Martín JL, Naves-Díaz M, Ruiz-Torres MP, Cannata-Andía JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46:121-8. [ Links ]
Acknowledgments:
This research study was made possible thanks to the FEIOMM funding grant awarded Natalia Carrillo López to attend the 33rd Congress of the ASBMR 2011 (San Diego, 2011). The work has also been partially funded with the help of the National Plan for R&D&I 2008-2011, State Plan for R&D&I 2013-2016, Carlos III Health Institute (ISCIII) - European Regional Development Fund/00415), Science, Technology and Innovation Plan 2013-2017 of the Principality of Asturias (GRUPIN14-028), Foundation for the Promotion in Asturias of Applied Scientific Research and Technology (FICYT), Reina Sofía Institute for Nephrological Research, Íñigo Álvarez de Toledo Renal Foundation, RETIC RedInRen of the ISCIII - European Regional Development Fund (RD06/0016/1013, RD12/0021/1023 and RD16/0009/0017), by the Asturian Society for Metabolic Research.
The handling of experimental animals was carried out in accordance with the current legal regulations (European Union Directive 2010/63/EU and Spanish Royal Decree 53/2013 of 1 February).
FundingThis research study was made possible thanks to the FEIOMM funding grant awarded Natalia Carrillo López to attend the 33rd Congress of the ASBMR 2011 (San Diego, 2011).
FundingThe work has also been partially funded with the help of the National Plan for R & D & I 2008-2011
FundingScience, Technology and Innovation Plan 2013-2017 of the Principality of Asturias (GRUPIN14-028)
FundingFoundation for the Promotion in Asturias of Applied Scientific Research and Technology (FICYT)
Received: June 23, 2017; Accepted: July 22, 2017











 texto en
texto en 


