Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.13 no.1 Madrid ene./mar. 2021 Epub 17-Mayo-2021
https://dx.doi.org/10.4321/s1889-836x2021000100006
REVIEW
Pathophysiology of osteoporosis in chronic inflammatory joint diseases
1Rheumatology Department. La Princesa University Hospital. Princesa Health Research Institute (IIS-Princesa). Madrid (Spain)
2UAM-Roche, EPID*-Future (*Diffuse Interstitial Lung Diseases). Autonomous University of Madrid (UAM). Madrid (Spain)
3Comprehensive Services for Health Management (SIGEMED). Madrid (Spain)
4Rheumatology Department. La Paz University Hospital. La Paz University Hospital's Research Institute (IdiPAZ). Madrid (Spain)
The immune system and the bone often share the same anatomical niches and spaces, as there is a close functional relationship between both of them. As a consequence, there is a constant interaction between them and a bidirectional flow of information between the immune cells and those of the bone tissue (osteoclasts, osteoblasts and osteocytes) often unknown, in which multiple inflammatory mediators and various growth and cell differentiation factors are involved. This leads to a very close interaction between inflammation and bone loss. In fact, osteoporosis (OP) is one of the most frequent systemic complications in chronic inflammatory diseases (CIDs). The prevalence of OP in CIDs depends on each pathological scenario. Rheumatoid arthritis (RA) is a paradigmatic disease which causes chronic inflammation, where the presence of OP is frequent and shows even prior to the appearance of the first symptoms of the RA. The pathogenesis of RA-associated OP is complex and includes the cooperation of multiple pro-inflammatory cytokines that promote osteoclastogenesis and inhibit bone formation. Tumor necrosis factor alpha (TNF-α) and different interleukins (IL), such as IL-1, IL-6 and IL-17, stand out among all, IL-6 having a relevant hierarchical role. In this study, we review the role of pro-inflammatory cytokines in bone and joint destruction in different CIDs, giving special emphasis to RA, as we set out the bases of possible pathways that open new therapeutic horizons in the their framework.
Key words osteoporosis; rheumatoid arthritis; psoriatic arthritis; ankylosing spondylitis; pathophysiology; interleukins; IL-6; treatment
CHRONIC INFLAMMATORY DISEASES
Chronic inflammation is a nonspecific response against aggressor agents mediated by the body’s immune system. In such a scenario, an infiltrate of predominantly mononuclear cells, such as lymphocytes, macrophages and plasma cells, is produced. Under certain conditions or when the aggressor agent persists, a sustainable accumulation and activation of immune cells occurs. Then, the secretion of cytokines, agents that prolong the life of lymphocytes and macrophages, is increased, what leads to chronic inflammation.
Inflammation is the main mechanism involved in bone destruction in chronic inflammatory diseases (CIDs)1, such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE), multiple sclerosis and/or inflammatory bowel disease (IBD). These diseases show a chronic systemic inflammation that can affect different organs, caused by an alteration of the immune system2.
One of the characteristics of CIDs is the common symptoms that patients present: malaise, fatigue, daytime sleepiness, weakness, nonspecific arthromyalgia, hyporexia, anxiety and low mood2.
Inflammatory joint diseases encompass more than 100 different and heterogeneous disorders that affect the joints and cause disability. However, RA and spondyloarthritis (SpA: AS, reactive arthritis, PsA and SpA associated with IBD) are the most frequent1.
RA is an autoimmune disease considered the prototype of destructive inflammatory arthritis and characterized by chronic inflammation of the synovium in multiple joints and tendon sheaths. The synovial membrane is the target organ where the immune system interferes with bone homeostasis, producing severe structural damage and bone destruction there where joint and peri-articular inflammation exist3-5. In fact, in patients with inflammatory rheumatic diseases, bone destruction occurs together with erosions, periarticular osteopenia and/or generalized osteoporosis (OP )1,4.
The cause of RA-associated OP has its origin in an alteration of bone remodeling, which is the common pathophysiological mechanism of both diseases. The loss of bone mass in RA can be periarticular or generalized. Periarticular loss, commonly called juxta-articular OP, affects the trabecular and cortical bone and is one of the first radiological manifestations. It can precede both the appearance of erosions and damage to the joint space6, and is easily detected on X-rays of the hands. Accelerated bone loss in the hands has been associated with the development of RA in patients suffering undifferentiated arthritis7 and presenting progressive joint disease in the hands and feet at the onset of RA8-10.
Another form of RA bone loss involves erosion of the marginal bone as a consequence of inflammation of the synovial membrane8. The erosion, generally irreversible, may begin before arthritis symptoms appear and is related to the severity of the disease and functional impairment1. Finally, and due to autoimmune mechanisms, RA usually produces generalized bone loss (systemic OP), inclusively in those regions of the skeleton located far from the inflamed joints8 even in the initial stages of the disease11.
THE BONE SYSTEM AND THE IMMUNE SYSTEM
The musculoskeletal system and the immune system closely interact in the homeostasis of hemopoietic and lymphopoietic cells, acting in the pathogenesis of CIDsassociated OP as well as in postmenopausal OP, but it remains to be explained how adaptive immune responses affect the bone tissue. However, recent evidence has revealed that the reverse is also true: bone cells regulate immune cells, a concept consistent with the established role of bone marrow in the development and homeostasis of the immune system6,12,13.
Due to its anatomical characteristics, both the in and out of the bone tissue are closely related to the immune system. In the inside, in the bone marrow, hematopoiesis occurs, so bone and immune cells locally work together in an indisputable way. At the outside, the skeleton is in direct contact with the periosteum, entheses, and juxta-articular bone, where it connects with determining structures that have a role in the joint destruction process that characterizes chronic inflammatory joint diseases (CIJDs)13.
Likewise, the immune system and bone tissue are connected to the general circulation by nutritional and periosteal vessels that cross the cortical bone, and, within the bone compartment, this connection is produced through fibrous enthesis junctions and calcified components of cartilage and fibrocartilage14.
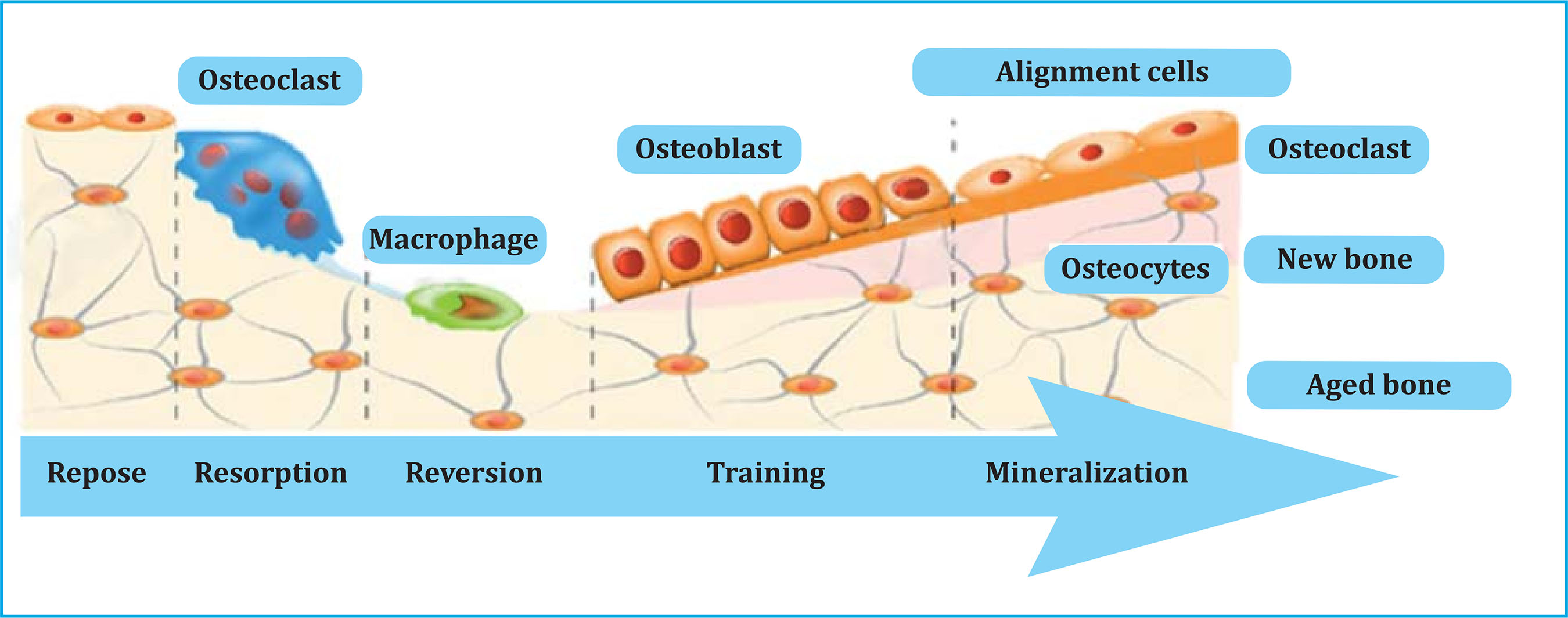
This permanent interaction between bone and immune system is of great importance in maintaining bone homeostasis and is also key in bone pathology. Throughout adult life, bone remodeling occurs in basic multicellular units (BMUs) or bone remodeling units, where osteoclasts reabsorb a certain amount of bone, and osteoblasts form the osteoid matrix and mineralize it to fill the previously created cavity (Figure 1). There are osteoclasts, macrophages, preosteoblasts and osteoblasts inside BMUs that are governed by a series of factors, both general and local, and allow the normal functioning of bone tissue and the maintenance of bone mass.
Bone cells interact with the immune system cells in the bone marrow development during growth and the healing of fractures. Apart from that, osteoblasts play an important role in controlling the renewal and differentiation of hemopoietic stem cells and B cells in places close to the endosteum14.
A series of substances synthesized by bone cells, immune system cells or bone marrow have an effect on bone growth and remodeling. The most important local factors are growth factors, cytokines, and bone matrix proteins (Table 1).
Table 1. Main mediators involved in bone remodeling
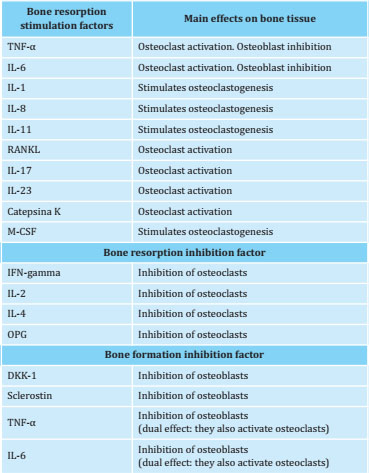
DKK-1: Dickkopf-1; IFN: interferon; IL: interleukin; M-CSF: macrophage colony stimulating factor; OPG: osteoprotegerin; RANKL: receptor activator of nuclear factor kappa B ligand (NF-κB); TNF: tumor necrosis factor.
Modified from ref. 5 (Llorente I et al. Front Med. 2020).
The musculoskeletal and the immune systems interact with each other, sharing molecules and generating a collaborative regulatory system called "osteoimmune system". The most representative and well-known molecule of this system is the receptor activator of nuclear factor kappa B ligand (RANKL), which fulfils multiple functions, both under physiological conditions and in conditions as different as RA and bone metastases. Based on current evidence showing great mutual dependence, it is accepted that the relationship between bone and the immune system does not develop by accident, but as a necessary consequence of evolution6.
RANKL expression in osteoblasts is stimulated by various factors or molecular mediators, such as the interleukin (IL) 1, IL-6, IL-11, IL-15, IL-17, TNF-α, prostaglandin E2, parathormone (PTH), calcitriol, interferon and glucocorticoids (GCs), and is suppressed by the transforming growth factor-β (TGF-β) (Table 1). On another note, the osteoprotegerin (OPG) expression is stimulated by the TGF-β, bone morphogenetic proteins (BMPs), interferon (IFN), IL-6, IL-11 and IL-13 and is inhibited by the PTH, IL-17, calcitriol and GCs. Estrogens inhibit RANKL production and increase OPG and TGF-β secretion (Table 1)5,13,15,16.
The immune and bone systems share, indeed, a wide range of regulatory mechanisms, and today we can assert this influence to be bidirectional, not only of the immune system on the bone, but also in the opposite direction6,13. In fact, in the bone marrow microenvironment, bone cells and immune system cells are so closely located that their interaction is logical17.
The RANK/RANKL/OPG system is the promoter of most of the factors that regulate bone resorption. It belongs to the group of proteins related to tumor necrosis factor α (TNF-α) and actively participates in the control of bone resorption and activation of osteoclasts15. Although the RANK/RANKL/OPG pathway remains the basis for understanding the coupling of the immune system cells with those of the bone system, some research suggests that there may be additional stimuli and unique pathways that act independently or in concert with RANK12.
Systemic inflammation in RA increases the production of inflammatory cytokines, such as TNF-α, IL-1, IL-6 and IL-17, which act on the RANK/RANKL/OPG system, activating osteoclastogenesis and increasing bone resorption, due to RANKL’s wide expression in synovial fibroblasts and in the T cells of inflamed joints of RA patients17. This abnormal activation of osteoclasts in the absence of equivalent levels of osteoblastic activity results in a generalized decrease in bone mass and a higher incidence of vertebral and non-vertebral fractures17,18.
The immune regulation of osteoclasts is closely related to the RA pathogenesis. There is evidence that RA bone destruction is primarily caused by the increased in osteoclast activity as a result of the activation of a unique subset of T helper cells, the T helper 17 cells (Th-17). These cells have a low production of IFN gamma (IFN-γ) and are capable of causing local inflammation through the production of pro-inflammatory cytokines3.
Mature Th-17 cells produce IL-17, IL-21, and IL-22, all of them cytokines with high pro-inflammatory activity. In RA, IL-17 produced by Th-17 cells exerts its osteoclastogenic effect by stimulating RANKL expression in synovial fibroblasts1,3. In immune precursor cells such as macrophages, IL-17 also stimulates the production of inflammatory cytokines, including TNF-α, IL-1? and IL-6, and get in contact with the RANK of osteoclast precursors, all this leading to the differentiation of osteoclasts, provoking them to migrate towards the marginal zone where erosions begin3,13. Furthermore, synovial cells stimulated by inflammatory cytokines also produce matrix-degrading enzymes that play an important role in articular cartilage destruction3.
ROLE OF INTERLEUKIN-6 (IL-6) IN RA AND OSTEOPOROSIS
IL-6 plays an essential role in the pathophysiology of RA and associated bone destruction. Through cell signalling, which can be initiated in the cell membrane or by soluble forms of its receptor, IL-6 acts both locally, promoting joint inflammation and destruction, and systemically, causing some of the extra-articular and systemic manifestations of the disease, including pain, fatigue, morning stiffness, anemia, depression, low mood and weight loss19-21.
IL-6 actions are mediated through the interaction between its non-signalling receptor-?, the IL-6 receptor (IL-6R?), with which it interacts first, and subsequently forms a bond with the transduction receptor of signals, the glycoprotein (gp) 13022. IL-6R? is expressed in hepatocytes, monocytes/macrophages, neutrophils, and some types of T cells19.
Through intracellular signalling by binding to its membrane receptor or by the classical pathway, IL-6 regulates normal processes related to the immune and neuroendocrine systems, hematopoiesis, bone metabolism, lipid and glucose metabolism and acute phase responses. When IL-6 binds to its soluble IL-6R receptor, it predominantly regulates systemic pro-inflammatory effects, including monocyte recruitment, macrophage differentiation, and T cell recruitment and differentiation. Its bond with cell membrane’s gp130 prolongs its life average, which is why elevated IL-6 values are observed in the serum and synovial fluid of these patients19.
Apart from this, IL-6 is an effective stimulator of osteoclast-induced bone resorption and is essential for the pathogenesis of bone loss in the context of chronic inflammation, as occurs in other pathologies such as IBD22. Elevated IL-6 values in patients with RA produce an increase in osteoclastogenesis and an imbalance of bone remodeling in favor of resorption, which leads to a generalized bone mass loss and, secondarily, osteoporosis22.
In the preclinical state of RA, IL-6 binds to various cell lines and causes neutrophil migration to the joints, contributing to the development of chronic inflammation, impaired B and T cell differentiation, and angiogenesis. Subsequently, hepatocytes are stimulated to produce acute phase reactants such as C-reactive protein, fibrinogen, and serum amyloid A19,20.
In summary, IL-6 is an essential mediator in the pathogenesis of RA, acting indirectly on the bone and controlling the inducing effects of TNFα and IL-1 bone resorption. IL-6 increases RANKL production, induces the RANKL mRNA expression and increases bone resorption through RANK/RANKL/OPG interaction. The resulting bone erosion and cartilage destruction, together with inflammation and thickening of the synovial membranes, cause the development of inflammatory pannus that causes irreversible damage to the joint19. Therefore, IL-6 inhibition is an excellent resource in the treatment of RA that minimizes joint and bone damage. IL-6 inhibitors target both IL-6 ligand and IL-6R19,23,24.
SECONDARY OSTEOPOROSIS CAUSED BY THE TREATMENT OF CIDS
The iatrogenesis produced by GCs also plays a relevant role in OP associated with CIDs25,26. In fact, the GC treatment used in RA, IBD and in 50% of premenopausal women with SLE is the most frequent cause of secondary OP and the first cause of OP in the population under 50 years of age27, mainly caused by the inhibition of bone formation, provoked by a decrease in the number and activity of osteoblasts and because GCs favor osteocyte apoptosis, and primarily due to abnormally activated osteoclastogenesis. GCs block the action of vitamin D in the absorption of calcium27. Patients with RA have a risk of vertebral and hip fracture 2 to 3 times higher than the general population of the same age and sex27. Furthermore, the dose and time of exposure to GC are keys to the risk of fracture25.
In RA, the coexistence of comorbidities is frequent and is related to the disease itself, inflammatory activity or treatment, resulting in an increase in physical disability. The decrease in physical activity that sometimes leads to prolonged immobilization periods also induces bone and muscle mass losses ("typical sarcopenia in RA").
OP TREATMENT IN PATIENTS WITH RHEUMATOID ARTHRITIS
In RA patients it is advisable to periodically evaluate the risk of fracture using fracture risk scales such as FRAX® (Fracture Risk Assessment Tool; https://www.sheffield.ac.uk/FRAX/tool.aspx?lang=sp) and/or periodic determination of bone mineral density (BMD) by dual X-ray densitometry (DXA). This recommendation is even more important in patients older than 50 years of age, suffering severe RA and/or who have received prolonged treatment with GCs28.
The main objective of the treatment of primary OP or as comorbidity of RA is fracture prevention29,30. The action of the treatment can be antiresorptive or bone-forming. The most widely used antiresorptive drugs are oral bisphosphonates and denosumab. Teriparatide is the treatment of choice when bone-forming treatment is to be started31.
To our knowledge, no randomized controlled trials with bisphosphonates have been published regarding patients with RA-associated OP with fracture as primary endpoint, but only regarding patients with GC-induced RA and OP32. In these patients, bisphosphonates prevent bone loss in the lumbar spine and femoral neck and reduce the risk of vertebral fracture after 24 months of treatment, but have no effect on the prevention of non-vertebral fractures33.
Although bisphosphonates are the first-line treatment for OP, denosumab has demonstrated its antiresorptive efficacy in patients with primary and secondary OP34. Denosumab is a human monoclonal antibody (IgG2) that targets and binds with high affinity and specificity to RANKL, preventing the activation of its receptor, RANK, on the surface of osteoclasts and their precursors. By preventing the RANKL/RANK interaction, osteoclast formation, function, and survival are inhibited, which in turn causes decreased bone resorption in trabecular and cortical bone34. In a randomized and controlled trial, treatment with denosumab and calcium and vitamin D supplements significantly increased lumbar spine and total hip BMD after 6 and 12 months, reducing the risk of fracture and also reducing the radiological progression of arthritis in RA patients treated with methotrexate33.
Teriparatide acts as an anabolic drug by increasing bone formation, stimulating osteoblastogenesis and decreasing the osteoblasts and osteocytes apoptosis32. In clinical practice, it has been observed that teriparatide treatment reflects a significant increase in BMD and a decrease in vertebral fractures in RA patients under GCs treatment35. These results were endorsed by the same authors in an integrated analysis consisting of four observational studies under clinical practice conditions, in which a reduction in non-vertebral fractures was also observed. However, these results should be viewed with caution, since they are uncontrolled studies36.
EFFECT OF IL-6 INHIBITORY THERAPIES ON BONE LOSS DURING RA
As mentioned previously, one of the deleterious effects induced by RA chronic inflammation is the bone mass loss produced by the imbalance in bone remodeling in favor of resorption8. Likewise, the use of GCs in RA patients for more than three months increases the bone mass loss, especially trabecular mass loss, raising vertebral and hip fracture risk37. A more recent study concluded that the incidence of fractures in patients receiving GC treatment is even higher than is known, especially at the beginning of the treatment. Thus, an annual incidence of vertebral fracture of 5.2% was detected in patients in an early stage of the treatment, this incidence decreased to 3.2% in those under prolonged treatment38.
Treatment of RA with IL-6 antagonists is effective in controlling inflammatory activity, since this cytokine not only causes local inflammation, but also damage to bone structures due to its ability to stimulate the expression of the RANKL and osteoclastogenesis3. Sarilumab and tocilizumab are two biological drugs approved in Spain for the treatment of RA. Their mechanism of action is based on blocking the IL-6 receptor.
The effect of tocilizumab and sarilumab on the biochemical markers of bone remodeling, both formation and resorption, has been analyzed in some clinical trials. In the MONARCH study, monotherapy with sarilumab compared with that with adalimumab, achieved a significantly greater reduction in the RANKL biomarker for bone resorption, and a greater increase in the markers of procollagen type 1 N-terminal propeptide (P1NP) and osteocalcin39. In this and other trials, it has been shown that the decrease in RANKL levels and in RANKL/OPG ratio begins in the early stages of the treatment (week 2 after the start of the treatment with sarilumab), and that the decrease is maintained and even progresses during the 24 weeks of the study39-41.
Tocilizumab treatment over one year has not shown some significant changes in BMD in patients with normal baseline values, but in those with osteopenia42. After two years of treatment, tocilizumab has shown a significantly increased BMD in the femoral neck in patients with positive cyclic anti-citrullinated peptide antibodies (ACPA)43. Regarding bone remodeling biomarkers, tocilizumab significantly increases bone formation, achieving a 25% reduction in the carboxy-terminal telopeptide of type I collagen (CTX-I)/osteocalcin ratio after 16 weeks of treatment44, a small decrease (<15%) in the CTX-I and cross-linked carboxy-terminal telopeptide of type I collagen resorption biomarkers generated by matrix metalloproteinases (ICTP)45 at 24 weeks, and a significant increase in osteocalcin levels in 100% of patients at the end of the 52 weeks of treatment46.
All these data together suggest that the specific blocking of IL-6 could produce a direct anti-osteoporotic effect which is added to its indirect beneficial effects, such as the clinical control of the disease activity or the reduction, and even withdrawal of the systemic inflammation. Anti-TNF agents have also shown some efficacy in reducing systemic bone loss in RA8,27.
SECONDARY OSTEOPOROSIS CAUSED BY ANKYLOSING SPONDYLITIS AND OTHER CIDS
25% of patients with AS present OP and an increased risk of vertebral and non-vertebral fractures. Although bone loss depends on multiple factors, the effect of proinflammatory cytokines (TNF-α, IL-1 and IL-6) on osteoclast activation appears to be one of the main ones. In advanced stages of the disease, the decrease of BMD and the occurrence of fractures are also influenced by mechanical factors due to immobility, and spine stiffness.
The assessment of BMD in AS in the spine using traditional DXA is more difficult to carry out because of the appearance of ossification/syndesmophytes, especially in late stages of the disease, which can overestimate the assessment of the subject's calcium mineral content, although bone loss has been detected in other anatomical regions such as the hip even in the initial stages of the disease47. A decrease in bone mass has been also detected in the spine using other techniques, complementary to DXA, such as DXA assessment using lateral projection, less sensitive to artifacts48, or by applying the trabecular bone score (TBS)49, which allows visualization of the bone micro-texture of the vertebral body avoiding the addition of calcium that supposes the presence of syndesmophytes or other juxta-vertebral ossifications, and which is a good predictor of clinical vertebral fracture and major osteoporotic fracture in patients with AS, regardless of the FRAX®49.
The treatment with TNF-α inhibitors, the most widely used biological treatment, in these patients, not only reduces the inflammatory activity of the disease, but also improves the quantification of remodeling biomarkers and increases BMD50, although it is not yet clear that they reduce the incidence of new fractures51.
The prevalence of OP and fracture risk in patients with psoriasis and PsA is a widely debated issue, still unclear at the present time. Traditionally, there is a higher prevalence of OP in patients with psoriasis and PsA, when compared with the control population52,53. Regarding fractures, in a population-based study carried out by Ogdie et al. patients with psoriasis and PsA were reported to have a higher risk of fractures, with an adjusted hazard ratio (HR) of 1.26 (1.06-1.27) in patients with PsA, while patients with severe or severe psoriasis would have increased risk of any type of OP fracture as well as vertebral fractures: adjusted HR of 1.26 (1.151.39) and 2.23 (1.54-3.22), respectively54.
However, other authors do not seem to find in patients with PsA a prevalence of OP higher than in the general population, except for those presenting more severe polyarticular involvement and poorer functional grade55,56.
Finally, SLE patients, a prototype of chronic systemic autoimmune disease, also have a higher incidence of OP and fractures than the general population, due to the confluence of several factors such as: prolonged treatment with GCs, use of anticoagulants and immunosuppressants, periods of transient amenorrhea suffered by many patients with SLE in flare-ups, vitamin D deficiency57 and low physical activity, in addition to the inflammatory activity of the disease caused by various cytokines and pro-inflammatory mediators58.
CONCLUSIONS
CIJDs frequently present associated OP, although with different prevalence and severity depending on the type of underlying disease. CIJDs include RA, AS and PsA. RA is the prototypical disease that appears together with chronic inflammation and OP and, therefore, the only one included in different fracture risk assessment scales such as FRAX®. In fact, RA is a disabling disease, frequently associated with localized and generalized OP, in approximately one third of patients. The incidence of OP in RA patients depends on multiple factors, such as disease severity, age, prolonged use of corticosteroids, sarcopenia, and periods of prolonged immobilization. IL-6 is a crucial pro-inflammatory cytokine that plays a relevant role in the pathogenesis of joint inflammation and RA-associated OP. Treatment with IL-6 neutralizing agents improves both the joint and systemic symptoms, as well as the associated OP.
AS and PsA are also chronic inflammatory diseases that are associated with OP to a lesser extent, at least in its early stages, and which involved molecular mechanisms are less understood. The use of anti-TNF drugs in these patients have increased BMD and improved bone remodeling biomarkers, although their effect on fractures is more doubtful, so longitudinal clinical studies are needed to corroborate these incipient findings.
In all patients with a diagnosis of CIDs, especially RA, BMD and the risk of fractures should be early assessed, in order to start preventive treatment, at least consisting of calcium and vitamin D supplements and/or to administer a basic anti-osteoporotic treatment to try to prevent this dreaded complication, especially in cases with a higher risk of fracture.
Acknowledgments
To patients with chronic diseases, for everything they convey and teach us every day.
REFERENCES
1 Coury F, Peyruchaud O, Machuca-Gayet I. Osteoimmunology of bone loss in inflammatory rheumatic diseases. Front Immunol. 2019;10:679. [ Links ]
2 Straub RH, Cutolo M, Pacifici R. Evolutionary medicine and bone loss in chronic inflammatory diseases – a theory of inflammation-related osteopenia. Semin Arthritis Rheum. 2015;45(2):220-228. [ Links ]
3 Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017; 97:1295-1349. [ Links ]
4 Goldring SR, Gravallese EM. Mechanisms of bone loss in inflammatory arthritis: diagnosis and therapeutic implications. Arthritis Res. 2000;2(1):33-37. [ Links ]
5 Llorente I, García-Castañeda N, Valero C, González-Álvaro I, Castañeda S. Osteoporosis in rheumatoid arthritis: dangerous liaisons. Front Med. 2020;7:601618. [ Links ]
6 Takayanagi H. Osteoimmunology-Bidirectional dialogue and inevitable union of the fields of bone and immunity. Proc Jpn Acad. 2020; Ser. B96(4): 159-169. [ Links ]
7 De Rooy DP, Kälvesten J, Huizinga TW, van der Helm-van Mil AH. Loss of metacarpal bone density predicts RA development in recent-onset arthritis. Rheumatology (Oxford). 2012;51(6):1037-1041. [ Links ]
8 Zerbini CAF, Clark P, Méndez-Sánchez L, Pereira RMR, Messina OD, Uña CR, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28:429-446. [ Links ]
9 Forsblad-d'Elia H, Carlsten H. Bone mineral density by digital X-ray radiogrammetry is strongly decreased and associated with joint destruction in long-standing rheumatoid arthritis: a cross-sectional study. BMC Musculoskelet Disord. 2011;12:242. [ Links ]
10 Wevers-de Boer KV, Heimans L, Visser K, Kälvesten J, Goekoop RJ, van Oosterhout M, et al. Four-month metacarpal bone mineral density loss predicts radiological joint damage progression after 1 year in patients with early rheumatoid arthritis: exploratory analyses from the IMPROVED study. Ann Rheum Dis. 2015;74(2):341-346. [ Links ]
11 Llorente I, Merino L, Ortiz AM, Escolano E, González-Ortega S, García-Vicuña R, et al. Anti-citrullinated protein antibodies are associated with decreased bone mineral density: baseline data from a register of early arthritis patients. Rheumatol Int. 2017;37(5):799-806. [ Links ]
12 Walsh MC, Takegahara N, Kim H, Choi Y. Updating osteoimmunology: regulation of bone cells by innate and adaptative immunity. Nat Rev Rheumatol. 2018;14(3):146-156. [ Links ]
13 Arboleya L, Castañeda S. Osteoinmunología: el estudio de la relación entre el sistema inmune y el tejido óseo. Reumatol Clin. 2013;9(5):303-315. [ Links ]
14 Geusens P, Lems WF. Osteoimmunology and osteoporosis. Arthritis Res Ther. 2011;13:242-258. [ Links ]
15 Fiter Aresté J. Factores locales reguladores del metabolismo óseo. En: Arboleya Rodríguez L, Pérez Edo L, eds. Manual de Enfermedades Óseas. Madrid: Médica Panamericana; 2010. p. 27-33. [ Links ]
16 Pacifici R. Role of T cells in the modulation of PTH action: physiological and clinical significance. Endocrine. 2013; 44(3):576-582. [ Links ]
17 Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol. 2012;5:684-689. [ Links ]
18 Guañabens N, Olmos JM, Hernández JL, Cerdà D, Hidalgo Calleja C, Martínez López JA, et al; OsteoResSer Working Group of the Spanish Society of Rheumatology. Vertebral fractures are increased in rheumatoid arthritis despite recent therapeutic advances: a casecontrol study. Osteoporos Int. 2021 Jan 18. Online ahead of print. [ Links ]
19 Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther. 2020;7:473-516. [ Links ]
20 Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8(7):538-542. [ Links ]
21 Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2(5):247-256 [ Links ]
22 Edwards CJ, Williams E. The role of interleukin-6 in rheumatoid arthritisassociated osteoporosis. Osteoporos Int. 2010;21:1287-1293. [ Links ]
23 Dayer JM, Choy E. Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor. Rheumatology (Oxford). 2010;49(1):15-24. [ Links ]
24 Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020; 16(6):335-345. [ Links ]
25 Whittier X, Saag KG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 2016;42(1):177-189, x. [ Links ]
26 Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. 2019;30 (6):1145-1156. [ Links ]
27 Mirza F, Canalis E. Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173(3): R131-151. [ Links ]
28 Naranjo Hernández A, Díaz del Campo Fontecha P, Aguado Acín MP, Arboleya Rodríguez L, Casado Burgos E, Castañeda S, et al. Recommendations by the Spanish Society of Rheumatology on osteoporosis. Reumatol Clin. 2019; 15(4):188-210. [ Links ]
29 Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017; 69(8):1521-1537. [ Links ]
30 Lee TH, Song YJ, Kim H, Sung YK, Cho SK. Intervention thresholds for treatment in patients with glucocorticoid-induced osteoporosis: systematic review of guidelines. J Bone Metab. 2020;27(4):247-259. [ Links ]
31 Lespessailles E, Chapurlat R. High fracture risk patients with glucocorticoid-induced osteoporosis should get an anabolic treatment first. Osteoporos Int. 2020;31(10):1829-1834. [ Links ]
32 Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging. 2019;36:1061-1072. [ Links ]
33 Allen CS, Yeung JHS, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis (Review). Cochrane Database Syst Rev. 2016; 10:CD001347. [ Links ]
34 Agencia Española de Medicamentos y Productos Sanitarios. Denosumab. (Prolia). https://cima.aemps.es/cima.pdfs/es/ft/10618003/FT_10618003.pdf. [ Links ]
35 Langdahl BL, Silverman S, Fujiwara S, Saag K, Napoli N, Soen S, et al. Realworld effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone. 2018;116:58-66. [ Links ]
36 Silverman S, Langdahl BL, Fujiwara S, Saag K, Napoli N, Soen S, et al. Reduction of hip and other fractures in patients receiving teriparatide in real-world clinical practice: Integrated analysis of four prospective observational studies. Calcif Tissue Int. 2019;104:193-200. [ Links ]
37 Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323-328. [ Links ]
38 Amiche MA, Albaum JM, Tadrous M, Pechlivanoglou P, Lévesque LE, Adachi JD, et al. Fracture risk in oral glucocorticoid users: a Bayesian meta-regression leveraging control arms of osteoporosis clinical trials. Osteoporos Int. 2016;27:1709-1718. [ Links ]
39 Gabay C, Burmester GR, Strand V, Msihid J, Zilberstein M, Kimura T, et al. Sarilumab and adalimumab differential effects on bone remodelling and cardiovascular risk biomarkers, and predictions of treatment outcomes. Arthritis Res Ther. 2020;22:70. [ Links ]
40 Boyapati A, Msihid J, Fiore S, van Adelsberg J, Graham NMH, Hamilton JD. Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of MOBILITY. Arthritis Res Ther. 2016;18:225. [ Links ]
41 Gabay C, Msihid J, Zilberstein M, Paccard C, Lin Y, Graham NMH, et al. Identification of sarilumab pharmacodynamic and predictive markers in patients with inadequate response to TNF inhibition: a biomarker substudy of the phase 3 TARGET Study. RMD Open. 2018;4:e000607. [ Links ]
42 Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, Hatta K. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology (Oxford). 2011;53:900-903. [ Links ]
43 Chen YM, Chen HH, Huang WN, Liao TL, Chen JP, Chao WC, et al. Tocilizumab potentially prevents bone loss in patients with anticitrullinated protein antibody-positive rheumatoid arthritis. PLoS One. 2017;12:e0188454. [ Links ]
44 Karsdal MA, Schett G, Emery P, Harari O, Byrjalsen I, Kenwright A, et al. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RA-DIATE study (NCT00106522). Semin Arthritis Rheum. 2012;42:131-139. [ Links ]
45 Garnero P, Thompson E, Woodworth T, Smolen JS. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate. Arthritis Rheum. 2010; 62(1):33-43. [ Links ]
46 Bay-Jensen AC, Platt A, Byrjalsen I, Vergnoud P, Christiansen C, Karsdal MA. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum. 2014;43:470-478. [ Links ]
47 Cai PL, Yan YY, Wei W, Chen XS, Zhao J, Zhang ZK, et al. The bone mineral density of hip joint was reduced in the initial stage of ankylosing spondylitis? Medicine (Baltimore). 2020;99(8):e19132. [ Links ]
48 Fitzgerald G, Anachebe T, McCarroll K, O'Shea F. Measuring bone density in axial spondyloarthropathy: Time to turn things on their side? Int J Rheum Dis. 2020;23(3):358-366. [ Links ]
49 Richards C, Hans D, Leslie WD. Trabecular bone score (TBS) predicts fracture in ankylosing spondylitis: The Manitoba BMD Registry. J Clin Densitom. 2020;23(4):543-548. [ Links ]
50 Gulyás K, Horváth Á, Végh E, Pusztai A, Szentpétery Á, Pethö Z, et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2020;39(1):167-175. [ Links ]
51 Beek KJ, Rusman T, van der Weijden MAC, Lems WF, van Denderen JC, Konsta M, at el. Long-term treatment with TNF-alpha inhibitors improves bone mineral density but not vertebral fracture progression in ankylosing spondylitis. J Bone Miner Res. 2019;34(6):1041-1048. [ Links ]
52 Moltó A, Dougados M. Comorbidities in spondyloarthritis including psoriatic arthritis. Best Pract Res Clin Rheumatol. 2018;32(3):390-400. [ Links ]
53 Ocampo D V, Gladman D. Psoriatic arthritis. F1000 Res. 2019;8: F1000 Faculty Rev-1665. [ Links ]
54 Ogdie A, Harter L, Shin D, Baker J, Takeshita J, Choi HK, et al. The risk of fracture among patients with psoriatic arthritis and psoriasis: a populationbased study. Ann Rheum Dis. 2017; 76(5):882-885. [ Links ]
55 Gulati AM, Michelsen B, Diamantopoulos A, Grandaunet B, Salvesen Ø, Kavanaugh A, et al. Osteoporosis in psoriatic arthritis: a cross-sectional study of an outpatient clinic population. RMD Open. 2018;4(1): e000631. [ Links ]
56 Kwok TSH, Sutton M, Yang Ye J, Pereira D, Chandran V, Gladman DD. Prevalence and factors associated with osteoporosis and bone mineral density testing in psoriatic arthritis. Arthritis Care Res (Hoboken). 2020 Dec 16. Online ahead of print. [ Links ]
57 Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford). 2008;47(6): 920-923. [ Links ]
58 Ruaro B, Casabella A, Paolino S, Alessandri E, Patané M, Gotelli E, et al. Trabecular bone score and bone quality in systemic lupus erythematosus patients. Front Med (Lausanne). 2020; 30;7:574842. [ Links ]
Received: January 31, 2021; Accepted: February 26, 2021











 texto en
texto en